Our focus areas
Our theranostic approach is focused on urologic oncology, neuro-oncology (glioma), musculoskeletal oncology (sarcoma) and hematologic oncology. These represent markets with clear opportunities for improved oncology approaches and high unmet patient need.
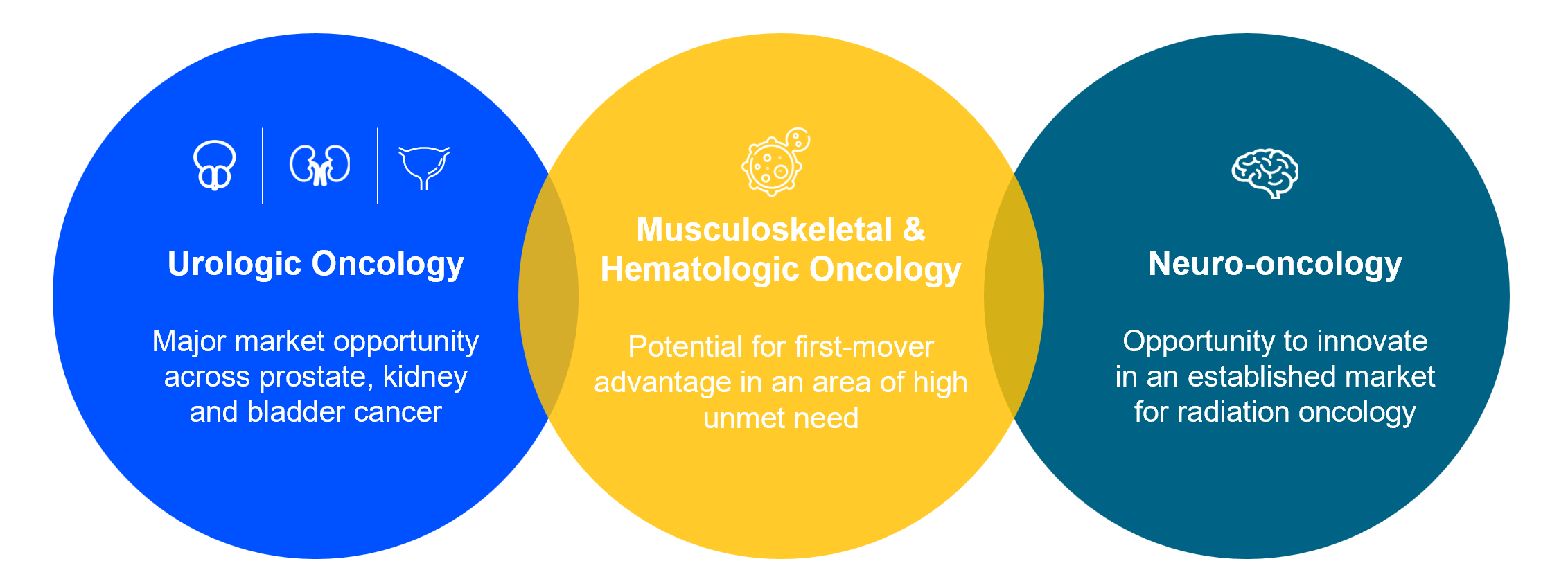
Urologic oncology
Prostate cancer
Our goal is to unlock the full potential of PSMA-targeted therapies.
The need
In 2022, the global incidence of prostate cancer was estimated to be 1,349,000, and this is expected to reach approximately 1,455,000 by 20271.
Our approach
Our prostate cancer programs target PSMA – a protein that is overexpressed on the surface of prostate cancer cells and is low or absent on most normal healthy cells. Imaging with targeted radiation can identify prostate cancer wherever it is in the body and help guide patient treatment. The PSMA receptor is expressed in over 80% of prostate cancer tumors. This expression of PSMA provides a specific target to design therapeutic and diagnostic agents for the treatment and imaging of prostate cancer.
Our portfolio
Therapeutic
TLX591 (177Lu rosopatamab tetraxetan) is our investigational radio antibody-drug conjugate (rADC) directed at PSMA. We are evaluating the efficacy and safety profile of TLX591 in the ProstACT series of clinical trials in prostate cancer, from first recurrence to advanced metastatic disease. In 2024, we dosed the first patients in ProstACT GLOBAL, a multicenter, international Phase 3 study to investigate and confirm the benefits and risks associated with TLX591, when administered in combination with standard of care (SoC) and compared to SoC alone. The study consists of two parts: Part 1 Safety & Dosimetry Lead In; and Part 2 Randomized Treatment Expansion, with an overall target enrolment of ~500 patients. We expect to provide an update on Part 1 in H1 2025.
TLX592 (64Cu/225Ac-RADmAb®) is our investigational next-generation targeted alpha therapy (TAT) based on our proprietary RADmAb® engineered antibody technology. The Phase 1 CUPID trial evaluated copper-64 (64Cu) labelled TLX592 in patients with advanced prostate cancer and delivered successful pharmacology and biodistribution proof-of-concept in 2024, prior to commencing therapeutic studies with actinium-225 (225Ac).
Precision medicine
Illuccix® (68Ga-PSMA-11), also referred to as TLX591-CDx in some territories where approval has not yet been granted, is our preparation for imaging prostate cancer with PET. It is currently approved in the U.S., Australia, Canada, Denmark and the United Kingdom, and is in national approval review in Europe following a positive decentralized procedure opinion from BfArM2. Sales of Illuccix® have been the primary source of revenue for Telix in 2024.
Gozellix3 (TLX007-CDx) – In 2024, Telix submitted an NDA for a new PSMA imaging product with a considerably extended geographic distribution radius from the nuclear pharmacy compared to currently approved gallium-68 (68Ga) based agents including Illuccix®. Its innovative properties are designed to facilitate more flexible production and further enhance patient access to PSMA-PET imaging and the clinical benefits of 68Ga imaging to underserved populations across the U.S. TLX007-CDx was accepted for filing by the FDA with a PDUFA4 goal date of 24 March 2025.

Navigating the reimbursement landscape
A key development in November 2024 was the announcement by the U.S. Centers for Medicare & Medicaid Services (CMS) that it will change the way it pays for specialised diagnostic radiopharmaceuticals. For Medicare fee-for-service patients in the Hospital Outpatient Prospective Payment System (OPPS), products that have gone beyond the transitional pass-through payment period (pass-through) will now be paid for separately by CMS. In 2025, these payments will be calculated on Mean Unit Cost (MUC), derived from hospital claims data, and applied to any specialised diagnostic radiopharmaceutical without pass-through status and with a threshold per-day cost greater than US$630.
For physicians in the hospital outpatient setting, it means purchasing decisions can be made based on the latest clinically significant diagnostic tools and evidence of utility, and not purely on reimbursement structure.
Under the changes announced by CMS, Illuccix® will remain eligible for reimbursement after July 2025, when its pass-through status is set to end. It should be noted that this funding model could be subject to further review under the new U.S. government.
Telix’s focus on product lifecycle management and innovation means that we will also have a new addition to our PSMA imaging franchise, Gozellix3, which is expected to attain pass-through status in mid-20255.
Kidney (renal) cancer and other cancers expressing CAIX
Our goal is to pioneer new theranostic approaches in kidney and other cancers expressing the biomarker CAIX where there is a significant unmet medical need.
The need
In 2022, the global incidence of kidney cancer was 434,8401. Clear cell renal cell carcinoma (ccRCC) is the most common and aggressive subtype of malignant kidney tumor, at 80-90%, and survival can depend on how early it is detected.
There are unmet needs for improvements in the diagnosis of ccRCC from indeterminate renal masses, and the staging of advanced disease through more accurate and specific imaging techniques. Despite the transformative impact of immunotherapies on the prognosis of patients with metastatic kidney cancer, a considerable number of patients fail to respond adequately to these therapies and eventually progress.
Our approach
Our target for kidney cancer is CAIX; a scientifically validated target in ccRCC, which is the most prevalent and aggressive form of kidney cancer. CAIX is a cell surface protein highly expressed in ccRCC and in many other solid tumors in the hypoxic tumor micro--environment. To target CAIX, we use a monoclonal antibody – girentuximab – which has been designed to have a high degree of selectivity and affinity for the target and is cleared from the body by the liver. The lack of kidney excretion is an advantage for patients with primary kidney disease. We believe the target profile and the properties of girentuximab make the ccRCC phenotype promising as the first therapeutic indication for TLX250.
Our portfolio
Therapeutic
TLX250 (177Lu-girentuximab) is our investigational rADC therapy for the treatment of advanced metastatic kidney cancer. It is being evaluated in two Phase 2 IITs in first and second-line kidney cancer, in combination with checkpoint inhibitors, and in STARSTRUCK2, a company-sponsored Phase 1 trial in combination with a Merck KGaA (Darmstadt, Germany) DNA-dependent protein kinase (DNA-PK) inhibitor candidate, peposertib.
The combined diagnostic and therapeutic potential of girentuximab may also extend into other cancers that significantly express CAIX, including certain Von Hippel Landau (VHL)-induced cancers, ovarian cancer, triple-negative breast cancer (TNBC) and bladder cancer. We have observed encouraging preliminary clinical data in TNBC and bladder cancer.
TLX252 (225Ac-girentuximab) is our investigational next-generation TAT that we are developing as a potential complement to the TLX250 (beta) program in ccRCC. TLX252 has demonstrated pre-clinical proof-of-concept and a first-in-human study is currently being planned.
Precision medicine
TLX250-CDx (Zircaix3, 89Zr- girentuximab) is a PET diagnostic imaging agent for the characterization of renal masses as ccRCC. Telix completed its U.S. BLA for TLX250-CDx PET imaging of ccRCC in December 2024. We continue to target a U.S. commercial launch in H2 2025, pending acceptance of the BLA for filing, and regulatory approval.
ZIRCON trial featured in The Lancet Oncology
Results from the Phase 3 ZIRCON trial, published in The Lancet Oncology, report that TLX250-CDx is highly accurate in detecting and characterizing ccRCC in patients with indeterminate renal masses (IRMs)4.
The paper’s authors explain that small masses in the kidney are increasingly being detected incidentally when patients undergo routine abdominal imaging, contributing to “an era of gross overtreatment”. Up to 30% of patients undergo unnecessary surgery, removing masses that are later determined to be benign. If confirmed, however, ccRCC is the most common and aggressive form of kidney cancer and delays in diagnosis can significantly reduce survival rates.
In this peer-reviewed paper, Professor Brian Shuch (University of California, Los Angeles) and colleagues, reported results from the prospective, open-label, multicentre, Phase 3 trial. They reported sensitivity of 86% and specificity of 87%, and positive predictive value of 93% in patients with cT1 IRMs (≤7cm). The authors concluded that TLX250-CDx “has a favourable safety profile and is a highly accurate, non-invasive imaging modality for the detection and characterisation of ccRCC, which has the potential to be practice changing.”
You can read the full article here: www.thelancet.com/journals/lanonc/article/PIIS1470-2045(24)00402-9/fulltext
Expanded access and compassionate use programs are active at more than 30 sites in the U.S.5, Europe and Australia. New studies were also launched exploring indication expansion for staging and recurrence, and surveillance6.
The Phase 3 ZIRCON-CP registration study in China dosed the first patients in November 20247 and continues to recruit well.
Bladder cancer
Our goal is to leverage a novel mode of action to improve outcomes for patients with both localised and disseminated, advanced disease.
The need
In 2022, the global incidence of bladder cancer was 614,2981, making it the ninth most common cancer worldwide and the sixth most common cancer in men.
The standard treatment for early-stage bladder cancer is endoscopic surgery. For advanced disease, more invasive surgery or radiation therapy is combined with chemotherapy or immunotherapy, which is associated with significant ill health and life-long impacts on quality of life. With few effective therapeutic options and the risk of complete cystectomy (bladder removal), a more targeted treatment approach is urgently needed.
Our approach
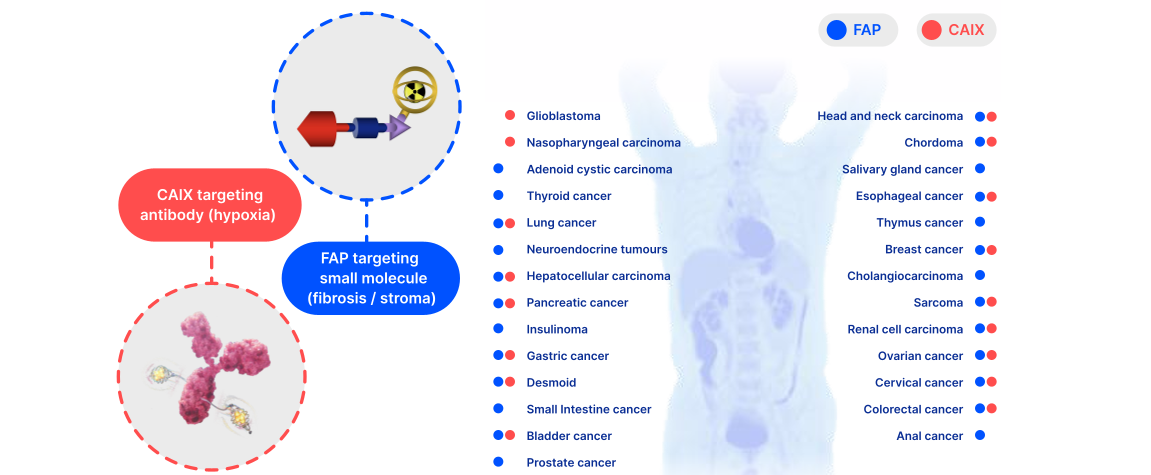
Our bladder cancer programs target CAIX and fibroblast activation protein (FAP), two well-validated targets with pan-cancer potential. This complementary approach targeting hypoxia and fibrosis is a potential “double hit” at the tumor micro-environment, with the potential to provide a synergistic benefit when combined with other systemic therapies like immuno-oncology.
Our portfolio
We have observed encouraging preliminary clinical data in bladder cancer with our CAIX-targeting TLX250 platform, and in November 2024, we announced an expansion of our theranostic pipeline with the in-licensing of a portfolio of next-generation FAP-targeting assets8. Our FAP development program will initially focus on the treatment of bladder cancer, complementing our existing successful urology franchise. We also plan to explore the potential for our FAP portfolio in a range of solid tumors, as many cancers are known to express this target either in the tumor micro-environment, like breast cancer, or directly on malignant lesions, like sarcomas. Our lead incoming therapeutic candidate has been administered in over 500 patients in a compassionate use setting covering multiple tumor types.
Neuro-oncology
Brain cancer (glioma)
Our goal is to improve the treatment options for patients with glioma based on a theranostic approach.
The need
In 2022, the global incidence of brain and nervous system tumors was 321,7311. Gliomas make up approximately 30% of all brain and central nervous system (CNS) tumors and 80% of all malignant brain tumors.
Glioblastoma (GBM) – the most aggressive sub-type of glioma – has a poor prognosis, primarily due to there being few effective treatment options, with a median survival from initial diagnosis typically in the range of 12-15 months. The mainstay of treatment for GBM is surgical resection, followed by combined radiotherapy and chemotherapy. Despite such treatment, recurrence occurs in almost all patients.
Our approach
Our brain cancer program targets two membrane transport proteins known as large amino acid transporter 1, and large amino acid transporter 2 (LAT1 and LAT2): validated targets that are highly expressed in several solid tumors, including malignancies of the CNS.
We believe the LAT1 and LAT2 receptors, expressed on both sides of the blood-brain barrier (BBB), are suitable targets for the delivery of radiation to both primary CNS malignancies and metastases from non-CNS cancers such as lung and breast cancer. As such, we see several potential indications for theranostic radiopharmaceuticals targeting LAT1 and LAT2.
Our portfolio
Therapeutic
TLX101 (131I-IPA) is our LAT1-targeting investigational therapy for patients with brain cancer. We use a small molecule for this therapy due to the need to cross the BBB. TLX101 has received orphan drug designation (ODD) in the U.S. and Europe for the treatment of glioma.
IPAX-1 trial featured in Neuro-Oncology Advances
Results of the Phase 1 IPAX-12 study were published in Neuro-Oncology Advances, confirming the safety and tolerability profile, and early efficacy of TLX101 therapy, in combination with external beam radiation therapy (EBRT) in recurrent GBM, the most common and aggressive form of primary brain cancer.
In the first peer-reviewed publication of the IPAX-1 study, Professor Josef Pichler (Kepler University Hospital, Austria) and colleagues reported that single or fractionated doses of TLX101 plus EBRT were associated with acceptable tolerability and specific tumor-targeting in patients with recurrent GBM. The authors explained that the study delivered encouraging preliminary efficacy data, demonstrating a median overall survival (OS) of 13 months from the initiation of treatment, or 23 months from initial diagnosis. The authors concluded that findings from the IPAX-1 study “support further investigation into the use of TLX101 plus EBRT, including its potential as a first line treatment”3.
You can read the full article here: www.academic.oup.com/noa/article/6/1/vdae130/7723438
We are currently evaluating TLX101 in the front-line (Phase 1)4 and recurrent (Phase 2)5 disease settings, where we have observed promising preliminary clinical evidence of anti-tumor effect and disease stabilization.
TLX102 (211At-APA ) is our investigational next-generation TAT that we are developing as a potential complement to the TLX101 (beta) program in GBM. TLX102 has demonstrated pre-clinical proof-of-concept, and a first-in-human study is in planning.
Precision medicine
Our investigational imaging agent, Pixclara6, also known as 18F-floretyrosine or 18F-FET, is a PET diagnostic agent designed to image cancerous lesions in the brain, and targets both LAT1 and LAT2.
Telix’s NDA was accepted by the FDA in October 2024 and granted a Priority Review, with a PDUFA goal date of 26 April 2025, paving the way for a U.S. commercial launch in 2025.
An expanded access program is active in the U.S.7
Musculoskeletal and hematologic oncology
Soft tissue sarcoma
Our goal is to leverage a successful pre-clinical program and established clinical safety profile to provide new treatment options for this disease, known to be susceptible to radiation.
The need
Soft tissue sarcoma (STS) is a complex disease encompassing a diverse group of relatively rare cancers, with more than 50 histological subtypes. Standard treatments for STS include surgery, radiation therapy and chemotherapy. For patients with advanced, unresectable or metastatic disease, treatment typically involves chemotherapy with single agents (e.g., doxorubicin) or anthracycline-based combination regimens. However, the prognosis for these patients remains poor, with treated patients with metastatic disease having a median overall survival of typically around 12 to 18 months.
Our approach
Our investigational products, TLX300 and TLX300-CDx employ antibody-directed targeted radiation for both therapeutic and diagnostic applications, respectively, against platelet-derived growth factor receptor alpha (PDGFRα), which is a tyrosine kinase receptor involved in fibrogenesis. We believe that the targeting of activated fibroblasts in the tumor micro-environment is a promising strategy to drive durable treatment responses in certain solid tumors. Eli Lilly and Company provided Telix with a licence for olaratumab, a naked antibody that was formerly marketed as Lartruvo®. We have repurposed olaratumab as a radiopharmaceutical.
Our portfolio
TLX300 has completed pre-clinical validation and we are enrolling patients on the Phase 1 ZOLAR trial1 of TLX300-CDx (89Zr-olaratumab) in patients with advanced, metastatic STS at the Melbourne Theranostic Innovation Centre (MTIC) in Melbourne, Australia. ZOLAR is a first-in-human, proof-of-concept and biodistribution trial that uses PET to evaluate olaratumab as a therapeutic radiopharmaceutical targeting platform.
Bone metastases and pain palliation
Our goal is to provide new, cost-effective solutions for treating pain associated with bone metastases, to improve the quality of life for patients.
The need
Most prostate cancer patients and 20% of breast cancer patients progress with bony (osteoblastic) lesions, which cause considerable pain. The current standard of care relies heavily on opioids, bisphosphonates and steroids, creating significant compliance and cost issues, and offering low quality of life for patients. Relief from pain due to bony metastases needs new, cost-effective solutions.
Our approach
We use a next generation chelating agent known as DOTMP1 to deliver a proprietary formulation of Samarium-153 (153Sm) radioisotope. DOTMP selectively targets sites of high bone mineral turnover, a known characteristic of bone metastases, and minimizes off-target migration.
Our portfolio
TLX090 (153Sm-DOTMP) is our novel bone-seeking product candidate that, unlike earlier products, avoids skeletal saturation and reduces the amount of free metal that would form colloids, resulting in lower marrow dose and improved clearance organ dosimetry. A single dose of TLX090 potentially offers up to four months of pain relief and quality of life.
A Phase 1 trial demonstrated highly targeted uptake in bone tumors, favorable safety profile and efficacy in reducing bone pain. In December 2024, a Type B pre-IND meeting with the FDA provided clear direction on the pathway to a Phase 2 bridging trial and we are now finalizing the protocol and study design based on FDA guidance.
Hematologic oncology
Our goal is to leverage existing and novel products to improve the imaging and treatment of blood- and bone-related cancers and infections.
The need
According to the Worldwide Network of Bone and Marrow Transplantation, there were approximately 90,000 first hematopoietic stem-cell transplantations (HSCT) performed in 20191, most often in patients with hematologic (blood) cancers, such as multiple myeloma or leukemia. Prior to HSCT, patients undergo a bone marrow conditioning (BMC) treatment; however the current standard of care - typically multi-drug chemotherapy regimens - can be highly toxic, and patients may not tolerate treatment. This creates an important unmet medical need for more tolerable BMC regimens.
Our approach
Our BMC program uses a monoclonal antibody called besilesomab to target distinct members of cluster of differentiation 66 (CD66), a family of receptors expressed on specific types of immune or blood cells that serve as attractive biomarkers for novel experimental conditioning radiopharmaceuticals.
Our portfolio
Therapeutic
Our product candidate for HSCT conditioning, TLX66 (90Y-besilesomab), has a broad clinical indication. It has been studied in acute myeloid leukemia (AML), multiple myeloma and systemic amyloid light-chain amyloidosis (SALA) through IITs. TLX66 has been granted ODD status in the U.S. and Europe.
Clinical data suggest TLX66 could be a well-tolerated (and therefore highly versatile) BMC agent, which could be utilized alone or in combination with reduced or high-intensity conditioning agents preceding HSCT.
Approximately 100 patients treated in several Phase 1 and 2 IITs of TLX66 in different hematological diseases requiring autologous or allogeneic stem cell transplantation have shown minimal uptake in non-hematopoietic organs such as liver, kidneys and gut.
We plan to evaluate TLX66 in a Phase 2 clinical trial as a BMC agent in patients with AML who are not suitable for conventional BMC regimes. We expect to submit an IND to the FDA for this trial and to commence the trial in 2025.
Precision medicine
The incidence of osteomyelitis (bone infection) is estimated to be as high as 21.8 cases per 100,000 persons per year2. The diagnosis of osteomyelitis is a challenge for diagnostic imaging and timely identification and localization of pathology can be of critical importance for appropriate management of patients.
TLX66-CDx (99mTc-besilesomab) is our commercial imaging agent for osteomyelitis. We previously out-licensed TLX66-CDx to Curium Pharma under the brand name Scintimun®. Under an agreement announced in January 2025, we have transferred the marketing and distribution rights back to Telix. We have identified significant potential to expand clinical utility, including as a companion patient selection and safety assessment tool for TLX66.
- Pharma Intelligence prostate cancer. Accessed February 2025.
- The German Federal Institute for Drugs and Medical Devices (Bundesinstitut für Arzneimittel und Medizinprodukte). Telix ASX disclosure 17 January 2025.
- Brand name subject to final regulatory approval.
- Prescription Drug User Fee Act.
- Subject to regulatory and CMS approval.
- Global Cancer Statistics 2022: GLOBOCAN survey. Published August 2024.
- ClinicalTrials.gov ID: NCT05868174.
- Brand name subject to final regulatory approval.
- Shuch et al. 2024.
- ClinicalTrials.gov ID: NCT06090331.
- ClinicalTrials.gov ID: NCT06447103.
- Telix media release 28 November 2024.
- Subject to completion of customary closing conditions.
- Global Cancer Statistics 2022: GLOBOCAN survey. Published August 2024.
- ClinicalTrials.gov ID: NCT03849105.
- Pichler et al. Neuro-Oncology Advances. 2024.
- ClinicalTrials.gov ID: NCT05450744.
- EudraCT Number: 2021-006426-43.
- Brand name subject to final regulatory approval.
- ClinicalTrials.gov ID: NCT06743100.
- ClinicalTrials.gov ID: NCT06537596.
- 1,4,7,10-Tetraazacyclododecane-1,4,7,10-tetrakis(methylenephosphonic acid)
- Neiderwieser et al. Haematologica. 2022.
- Kremers et al. J Bone Joint Surg Am. 2015.