We are a commercial-stage biopharmaceutical company focused on the development and commercialisation of therapeutic and diagnostic radiopharmaceuticals. We are headquartered in Melbourne, Australia with operations in the U.S., Europe (Belgium and Switzerland) and Japan. Our mission is to be the global leader in our field by combining therapeutic and diagnostic modalities for the benefit of patients, an innovative precision medicine concept generally referred to as 'theranostics'.
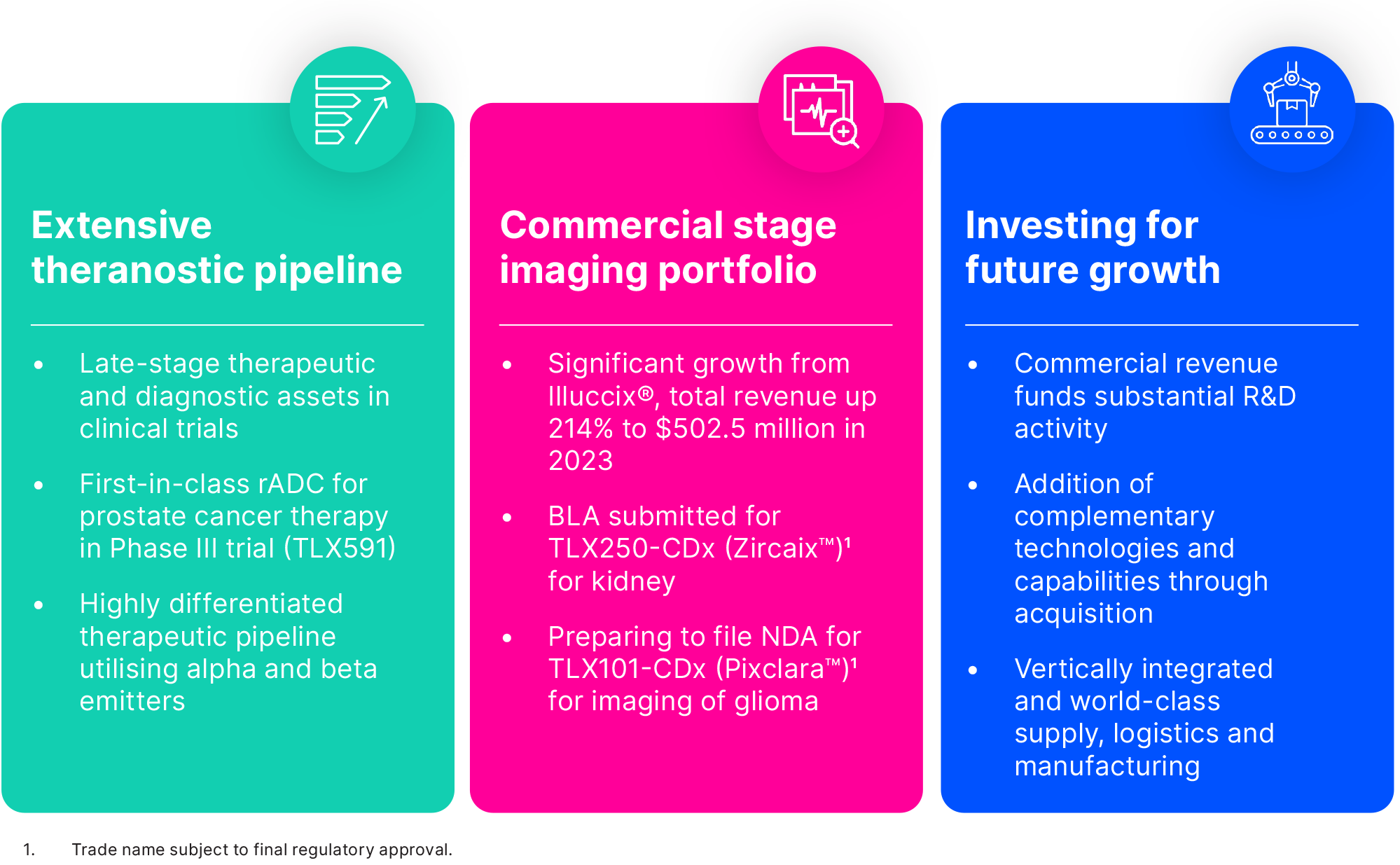
We have an extensive pipeline of theranostic radiopharmaceutical candidates, with a focus on urologic oncology (prostate and kidney), neuro-oncology (glioma), musculoskeletal oncology (sarcoma) and bone marrow conditioning. Our theranostic approach is intended to use imaging and therapy together to 'see and treat' cancer and rare diseases, to both better inform treatment decisions and deliver personalised therapy for patients.
Our therapeutic radiopharmaceutical platform harnesses the power of radioactive isotopes combined with multi-platform targeting agents to deliver targeted radiation directly to the tumour site. These therapies have the potential to be efficacious as stand-alone treatments or as complements to existing treatment modalities, addressing areas of high unmet medical need.
Underpinning our theranostic approach is the pairing of each therapeutic with a diagnostic imaging agent whereby two conjugates are used to target the same cell-surface receptor: one for detection, localisation or staging, and the other for selective destruction of target cancer cells. When used in tandem to plan and execute treatment, and then to assess response and monitor for progression, this approach allows the delivery of truly personalised therapy to patients.
Leading the field of radiopharmaceuticals
World-class innovation and manufacturing infrastructure

Our technology
Many existing therapies for cancer and rare diseases are non-selective and as a result can act against healthy tissue and vital organs while treating disease. Existing external beam radiation therapy (EBRT) approaches are effective but typically only deliver localised treatment and cause damage to surrounding tissue. Localised therapeutic approaches rely on the treating physician making assumptions about the extent of disease and can result in imprecise application of treatment. Treatments that miss small amounts of affected cells can lead to a recurrence of the cancer or disease.
Our radiopharmaceuticals are designed to deliver focused doses of radiation with precision targeting via an injection, regardless of where the cancer or disease is in the body.
We use a radioactive isotope as a payload, attached to a targeting agent – such as a small molecule or antibody – with an affinity for biomarkers on the surface of cancerous or diseased cells. Depending on the choice of radioisotope payload, either imaging or therapy can be delivered. This specificity of the targeting agent is designed to concentrate radiation at the tumour sites and to limit off-target tissue exposure.
Our clinical targets
We select clinical targets to pursue based on a deep understanding of radiation biology and radiopharmaceutical development. Our objective is to develop first-in-class or best-in-class theranostic products with a targeting agent and isotope-agnostic approach. We choose our targeting agents for the specific biological target and clinical application and then aim to optimise the radio-biology accordingly. We believe this approach allows for efficient drug development and gives us the ability to select the optimal targeting strategy and isotope for the tumour(s) being evaluated.
With the successful launch of Illuccix® – our commercially available PSMA-PET prostate cancer imaging agent – Telix has established itself as a leading innovator in radiopharmaceuticals.
Our extensive portfolio
Impacting the patient journey: From diagnosis to surgical intervention to therapy
In addition to our deep pipeline of theranostics, we aim to complement our theranostic product candidates with innovative nuclear medicine solutions spanning the patient treatment continuum from diagnosis, through surgical intervention, to therapy.
Our complementary portfolio approach is best exemplified by our offering in urologic oncology for the medical specialists managing the treatment of patients with prostate, kidney and bladder cancer. In prostate cancer our offering includes Illuccix®, surgical tools to guide cancer-detection, two therapeutic product candidates (TLX591 and TLX592) currently being evaluated in clinical trials, and a complementary AI platform currently in development to provide reader and clinical decision support. We are also building a similar portfolio of complementary products in kidney cancer and intend to expand this approach into other oncology indications.
Leadership in urologic oncology

We believe that therapeutic and diagnostic radiopharmaceuticals can become a fundamental pillar of cancer care that may deliver transformative survival and quality of life outcomes for patients, building upon recent practice-changing advances in immuno-oncology, targeted oncology and antibody-drug conjugates (as well as the advent of cell and gene therapies).
Clinical trials
At the time of this report, we have 18 clinical studies underway worldwide across a range of diseases. Some of these studies are funded directly by Telix, while others are funded in collaboration with leading cancer centres and commercial partners. This extensive investment puts Telix at the forefront of global innovation in theranostic drug development.
A full list of our active and completed clinical trials can be found at ClinicalTrials.gov

For most of our programs, particularly the prostate and kidney programs, we have generated extensive clinical data that demonstrate the potential for efficacy and good safety profile. We believe the targets and indications we are pursuing are well validated and are well suited for the delivery of therapeutic and diagnostic targeted radiation. The use of imaging to select patients for therapy is also a differentiated aspect of our commercial strategy.
It is our view that this precision medicine or theranostic approach may increase the potential of our therapeutic development programs, as patients can be selected for therapy with greater confidence that the drug target is sufficiently present to potentially confer therapeutic benefit. This may, in turn, lead to more streamlined and efficient clinical trials and enable improved patient outcomes.
Our core product development pipeline

Prostate cancer
Our goal is to unlock the full potential of PSMA-targeted therapies to help treat the approximately 1.4 million men worldwide who are diagnosed with prostate cancer every year.
In 2022, the global incidence of prostate cancer was estimated to be 1,349,000, and this is expected to reach approximately 1,455,000 by 2027.1
Our prostate cancer portfolio programs target PSMA – a protein that is overexpressed on the surface of prostate cancer cells and is low or absent on most normal healthy cells. PSMA has become a major breakthrough in the staging, treatment and management of prostate cancer. Imaging with targeted radiation can identify prostate cancer wherever it is in the body and help guide patient treatment. The PSMA receptor is expressed in over 80% of prostate cancer tumours.2 This expression of PSMA provides a specific target to design therapeutic and diagnostic agents for the treatment and imaging of prostate cancer.

Therapy
TLX591 (177Lu rosopatamab tetraxetan), is our investigational rADC directed at PSMA. We are evaluating the efficacy and safety profile of TLX591 in the ProstACT series of clinical trials in prostate cancer, from first recurrence to advanced metastatic disease.
Key TLX591 attributes include:
encouraging safety and tolerability data from the Phase I ProstACT SELECT trial3
promising evidence of efficacy demonstrated in Phase I-II studies, including up to 42.3 months median overall survival
high PSMA tumour antigen specificity with low rates of off-target organ exposure and an acceptable safety profile, and
two-dose regimen administered over 14 days, offering patient convenience with lower radiation exposure.
TLX592 (64Cu/225Ac-RADmAb®) is our investigational next-generation targeted alpha therapy (TAT) based on our proprietary RADmAb® engineered antibody technology. The Phase I CUPID trial4 is evaluating 64Cu-labelled TLX592 in patients with advanced prostate cancer, prior to commencing therapeutic studies with 225Ac.
Key TLX592 attributes include:
an engineered antibody vector designed for faster elimination from circulation than standard antibodies and slower elimination than small molecules that may result in side effects
a potentially reduced bone marrow residence time designed to mitigate the risk of haematologic toxicity, while retaining PSMA-mediated tumour localisation and exertion of cytotoxic activity, and
positive efficacy signal from in vivo animal studies.
We do not intend to develop diagnostic imaging applications with TLX592, and are using 64Cu to understand safety profile, pharmacology and dosimetry prior to use of an alpha-emitting isotope.
Imaging
Illuccix® (68Ga-PSMA-11) – also referred to as TLX591-CDx in some territories where approval has not yet been granted – is our preparation for imaging prostate cancer with PET. It is currently approved in the U.S., Australia and Canada.
Key Illuccix® attributes include:
a radiopharmacy distribution model with flexible scheduling
validated accuracy compared to other PSMA imaging agents, including lower rate of false positives and strong efficacy in patients with low disease burden, and
potential for expanded clinical utility based on guidelines and clinical research.
Our lead investigational therapy, TLX591, is a lutetium-labelled rADC that we believe has the potential to deliver a better efficacy and safety profile with a more efficient dosing regimen compared to existing small molecule products, including those commercially available and in clinical development.
TLX591 has been evaluated in 242 patients across eight clinical trials. A single-arm Phase II clinical trial of TLX591 reported a 42.3 month median survival in 17 patients with advanced metastatic castrate-resistant prostate cancer (mCRPC) when TLX591 was delivered under a fractionated dosing regimen.5 Median survival was 19.6 months at the lower dose level and was 27.8 months across both dose cohorts. In an area of high unmet medical need, this program is generating significant interest among clinicians and medical professionals.
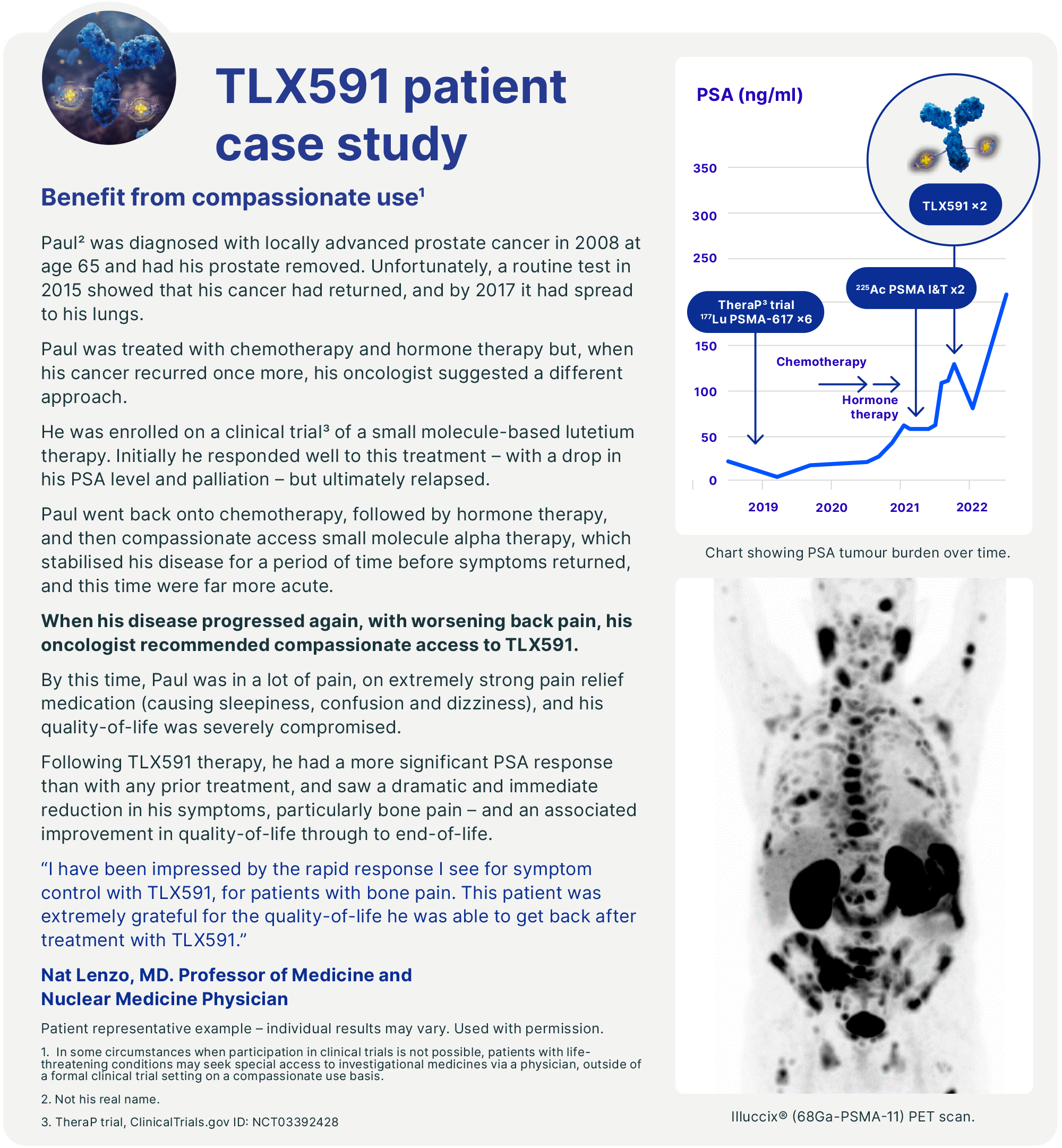
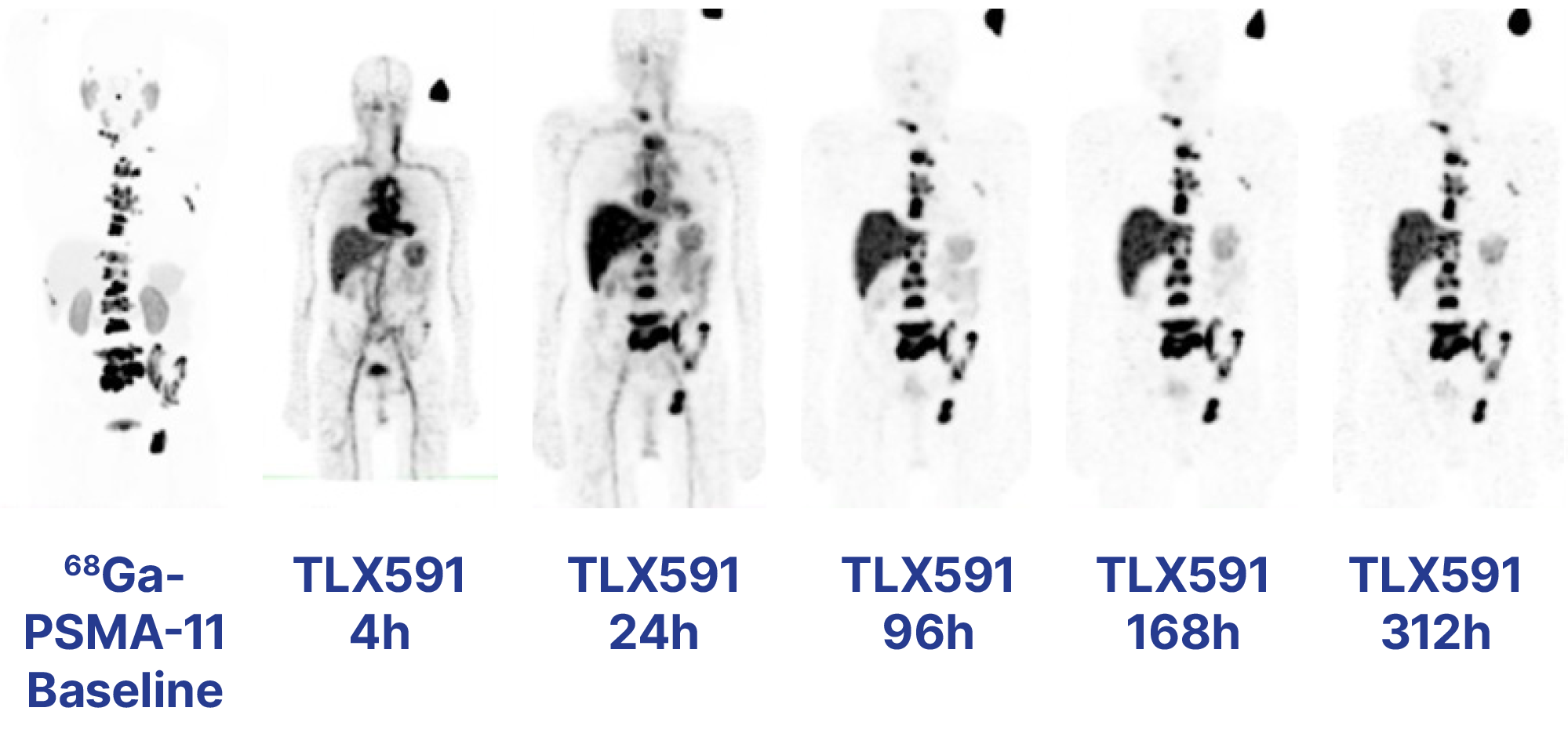
In November 2023, we initiated a randomised, multinational, multicentre, open-label Phase III trial – ProstACT GLOBAL – to evaluate TLX591 for the treatment of PSMA-positive mCRPC patients in combination with the standard of care (SoC) compared to SoC alone. We expect the final readout of the Phase I ProstACT SELECT trial - evaluating the safety and tolerability profile of TLX591 in combination with the SoC in mCRPC patients - in the second half of 2024.
We have built on our experience with TLX591 to develop TLX592 (225Ac-RADmAb®). TLX592 is our investigational next generation prostate cancer TAT and is the first clinical program based on our proprietary RADmAb® engineered antibody platform technology. Through the TLX592 program, we are exploring how the conjugation of an antibody vector with an alpha-emitting isotope might deliver a next-generation rADC with a different therapeutic profile. The Phase I CUPID trial is currently ongoing to evaluate 64Cu labelled TLX592 in patients with prostate cancer. To date, 11 patients with PSMA-avid disease – based on Illuccix® imaging – have been recruited to understand biodistribution and dosimetry before proceeding to a therapeutic study with an alpha-emitting isotope, actinium-225 (225Ac).

Our prostate cancer portfolio also includes Illuccix®, our commercially available gallium 68-labelled PSMA-PET imaging agent. The 'cold kit' format of Illuccix® enables rapid radiolabeling at room temperature with high radiochemical purity and production consistency, which is suited to the commercial and hospital radiopharmacy setting. Approved indications in the U.S. include staging of high-risk patients, identification of suspected recurrence, and selection for PSMA-directed lutetium therapy. We are also exploring potential future utilisation in additional indications through our lifecycle management program. These include monitoring progression in metastatic and non-metastatic castrate-resistant patients and monitoring response to PSMA-directed lutetium therapy.
Kidney (renal) cancer and other cancers expressing CAIX
Our goal is to pioneer new theranostic approaches in kidney and other cancers expressing the biomarker CAIX where there is significant unmet medical need.
According to the Global Cancer Statistics 2020: GLOBOCAN survey, in 2020, the global incidence of kidney cancer was 431,288.6 ccRCC is the most common subtype of malignant kidney tumours at 80-90%, and is one of the subtypes with the worst prognosis, where survival can depend on how early it is detected.7
Currently, there are unmet needs for improvements in the diagnosis of ccRCC from indeterminate renal masses, and the staging of advanced disease through more accurate and specific imaging techniques. Despite the transformative impact of immunotherapies on the prognosis of patients with metastatic kidney cancer, a considerable number fail to respond adequately to these and eventually progress.8
Our target for kidney cancer is CAIX; a scientifically validated target in ccRCC, which is the most prevalent and aggressive form of kidney cancer. CAIX is a cell surface protein that is highly expressed in ccRCC and in many other solid tumours in the hypoxic tumour microenvironment. Hypoxic tumour cells are characteristic of advanced disease with typically poor treatment outcomes. Hypoxic tumours are also typically more aggressive and less responsive to current treatments, particularly immunotherapies.

Therapy
TLX250 (177Lu-DOTA-girentuximab) is our investigational rADC therapy for the treatment of advanced metastatic kidney cancer.
Key TLX250 attributes include:
early clinical trials in patients with advanced ccRCC demonstrate promising safety profile and efficacy outcomes
animal models indicate that the combination of TLX250 with checkpoint inhibitor immunotherapies can improve therapeutic response, and
potential application in a range of cancers with unmet clinical needs that are known to express CAIX.
Imaging
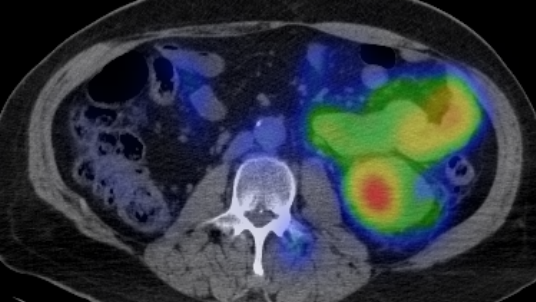
TLX250-CDx (89Zr-DFO-girentuximab, Zircaix™9) is our PET diagnostic imaging agent for the characterisation of renal masses as ccRCC. A BLA has been submitted to the FDA under a rolling review schema for marketing authorisation.
Key TLX250-CDx attributes include:
high affinity for CAIX, expressed in up to 94% of ccRCC and many hypoxic solid tumours, with low expression in normal tissue, and
positive results in Phase III ZIRCON trial10 and 'Breakthrough Designation' from the FDA.
A U.S. launch is targeted for 2024, with future expansion into the Europe and Asia Pacific regions, subject to regulatory approval.
To target CAIX, we use a monoclonal antibody – girentuximab – which has been designed to have a high degree of selectivity and affinity for the target and is cleared from the body by the liver. The lack of kidney excretion is an advantage for patients with primary kidney disease. We believe the target profile and the properties of girentuximab make the ccRCC phenotype promising as the first therapeutic indication for TLX250.
An increasing body of scientific evidence suggests low doses of targeted radiation can potentially overcome immune resistance.11 This approach, known as immunological 'priming', has the potential to render tumours more responsive to cancer immunotherapy.
TLX250 is being evaluated in two Phase II IITs in the first and second-line kidney cancer setting, in combination with checkpoint inhibitors,1213 and in a company-sponsored Phase I trial in combination with a Merck KGaA DNA-dependent protein kinase (DNA-PK) inhibitor candidate, peposertib.14
The combined diagnostic and therapeutic potential of TLX250 may also extend into other cancers that significantly express CAIX, including certain Von Hippel Landau (VHL)-induced cancers, ovarian cancer, triple-negative breast cancer (TNBC) and bladder cancer. We have observed encouraging preliminary clinical data in TNBC15 and bladder cancer.16
Our PET diagnostic imaging agent, TLX250-CDx (Zircaix™9), for the characterisation of renal masses as ccRCC, recently completed the pivotal Phase III ZIRCON trial. The trial met all primary and secondary endpoints and showed a 93% positive-predictive value (PPV) for ccRCC.17 We believe this demonstrated the ability of TLX250-CDx to reliably detect the clear cell phenotype and provide an accurate, non-invasive method for diagnosing ccRCC.
In December 2023, we submitted a BLA for TLX250-CDx to the FDA for imaging of ccRCC. The BLA was granted a rolling review process. Subject to regulatory approval, we aim to commercialise TLX250-CDx in the second half of 2024. If approved, TLX250-CDx will be the first targeted radiopharmaceutical imaging agent for kidney cancer in the U.S.. We believe TLX250-CDx is a natural follow-on product to Illuccix®, as it is targeted at the same clinician users, the urologist and urologic oncologist, and it leverages our existing commercial infrastructure.
Glioma (brain cancer)
Our goal is to improve the treatment options for patients with glioma, based on a theranostic approach.
According to the Global Cancer Statistics 2020: GLOBOCAN survey, in 2020, the global incidence of brain and nervous system tumours was 308,102.18 Gliomas make up approximately 30% of all brain and central nervous system (CNS) tumours and 80% of all malignant brain tumours.19
Glioblastoma (GBM) – the most aggressive sub-type of glioma – has a poor prognosis, primarily due to there being few effective treatment options, with a median survival from initial diagnosis of 12-15 months.20 The mainstay of treatment for GBM is surgical resection, followed by combined radiotherapy and chemotherapy. Despite such treatment, recurrence occurs in almost all patients.
Our brain cancer program targets two membrane transport proteins known as large amino acid transporter 1, and large amino acid transporter 2 (LAT1 and LAT2), validated targets that are highly expressed in several solid tumours, including malignancies of the CNS.
We believe that the LAT1 and LAT2 receptors, which are expressed on both sides of the blood-brain barrier (BBB), are suitable targets for the delivery of radiation to both primary CNS malignancies and metastases from non-CNS cancers such as lung and breast cancer. As such, we see several potential indications for theranostic radiopharmaceuticals targeting LAT1 and LAT2.

Therapy
TLX101 is our investigational therapy for the treatment of patients with brain cancer.
Key TLX101 attributes include:
Phase I/II IPAX-1 study21 met its primary endpoint of safety and tolerability profile of TLX101 and demonstrated encouraging tumour response in recurrent GBM
Phase I IPAX-2 study22 designed to extend TLX101 into the front-line setting for the first time, building upon experience in the recurrent setting
evidence of rapid clearance from the brain observed in the IPAX-1 trial, and
Orphan drug designation (ODD) in the U.S. and Europe, with potential to meet major unmet need.
Imaging
TLX101-CDx (Pixclara™9) is our investigational PET agent for imaging gliomas that is widely used in clinical research settings, including in our IPAX series of studies, as a complementary diagnostic agent to our TLX101 investigational therapy.
Key TLX101-CDx attributes include:
potential as a tool for the management of progression and treatment monitoring
ODD in the U.S., potential to meet major unmet need, and
widely used in Europe and recommended in joint guidelines for imaging of gliomas.23

TLX101 (131I-IPA) is our LAT1-targeting investigational therapy for patients with brain cancer. We are using a small molecule for this therapy due to the need to cross the BBB, the normal protective barrier that prevents many potential drug candidates entering the brain. TLX101 has received ODD in the U.S. and Europe for the treatment of glioma. We are currently evaluating TLX101 in the front-line (Phase I)24 and recurrent (Phase II)25 disease settings, where we have observed promising preliminary clinical evidence of anti-tumour effect and disease stabilisation.
Our investigational imaging agent, TLX101-CDx (Pixclara™9), also known as 18F-floretyrosine or 18F-FET, is a PET diagnostic agent designed to image cancerous lesions in the brain. TLX101-CDx has received ODD in the U.S. for the imaging of glioma and is currently being prepared for a NDA. TLX101-CDx targets both LAT1 and LAT2.
Soft tissue sarcoma
Our goal is to leverage a successful pre-clinical program and established clinical safety profile to provide new treatment options in this disease, known to be susceptible to radiation.
Soft tissue sarcoma (STS) is a complex disease that encompasses a diverse group of relatively rare cancers, with more than 50 histological subtypes. Standard treatments for STS include surgery, radiation therapy and chemotherapy. For patients with advanced, unresectable or metastatic disease, treatment typically involves chemotherapy with single agents (e.g., doxorubicin) or anthracycline-based combination regimens. However, the prognosis for these patients remains poor, with treated patients with metastatic disease having a median overall survival of around 12 to 18 months.26
Our investigational products, TLX300 and TLX300-CDx employ antibody-directed targeted radiation for both therapeutic and diagnostic applications, respectively, against platelet-derived growth factor receptor alpha (PDGFRα), which is a tyrosine kinase receptor involved in fibrogenesis. We believe that the targeting of activated fibroblasts in the tumour micro-environment is a promising strategy to drive durable treatment responses in certain solid tumours. Lilly provided us with a licence for olaratumab, a naked antibody that was formerly marketed as Lartruvo®. We have repurposed olaratumab as a radiopharmaceutical.
Therapy
TLX300 is our investigational therapy being developed for the treatment of patients with advanced or metastatic STS, administered in combination with doxorubicin.
Key TLX300 attributes include:
established clinical safety profile and favourable toxicology dataset, and
potential application in a range of other cancers (e.g. bone, brain, breast, lung, ovarian and prostate).
Imaging
TLX300-CDx is our investigational diagnostic, and a potential first imaging agent to specifically detect the presence of PDGFRα in patients with STS.
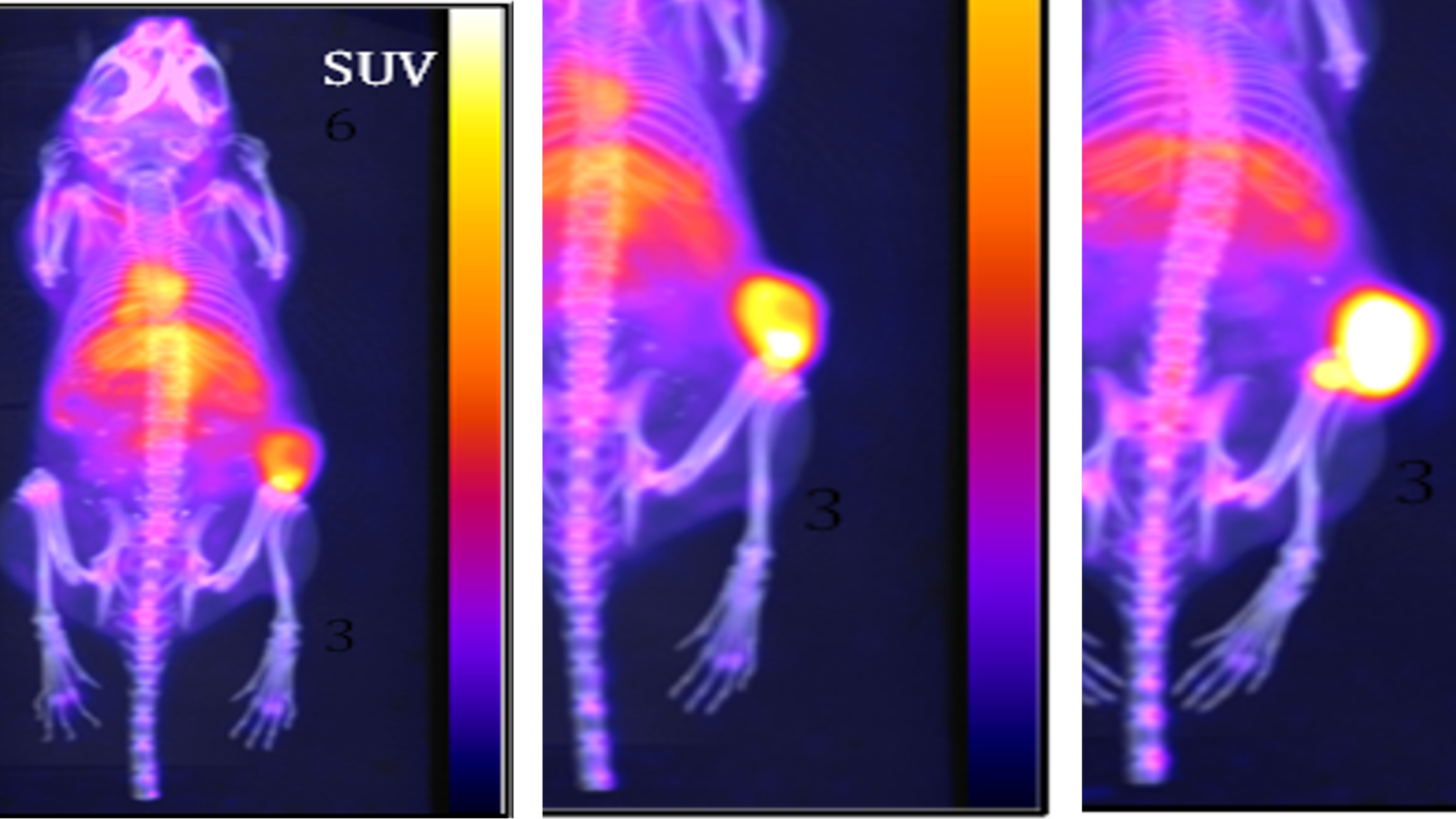
TLX300 has completed pre-clinical validation27 and we anticipate regulatory approval to initiate a proof-of-concept (PoC) targeting and biodistribution trial in Australia and New Zealand in the first half of 2024. We are developing TLX300 and TLX300-CDx (89Zr-DFOsq-olaratumab, including our proprietary DFO-squaramide chelator) as a theranostic pair targeting STS.
Bone marrow conditioning
Our goal is to develop reduced intensity conditioning regimes for patients unable to tolerate chemotherapy, addressing critical unmet medical need.
According to the Worldwide Network of Blood and Marrow Transplantation, in 2019 approximately 90,000 first haematopoietic stem cell transplantations (HSCTs) were performed, of which 47% were allogeneic.28
Prior to undergoing HSCT for the treatment of haematologic malignancies (blood cancers), patients undergo a BMC treatment. The current standard of care typically requires BMC with multi-drug chemotherapy regimens. However, these regimens are highly toxic, and patients may not tolerate treatment. This creates an important unmet medical need for more tolerable BMC regimens.
We are exploring the potential utility of targeted radiation in BMC to ablate bone marrow as part of a pre-conditioning regimen for bone marrow transplantation, novel stem cell therapies and gene therapies – each of which requires conditioning prior to treatment.
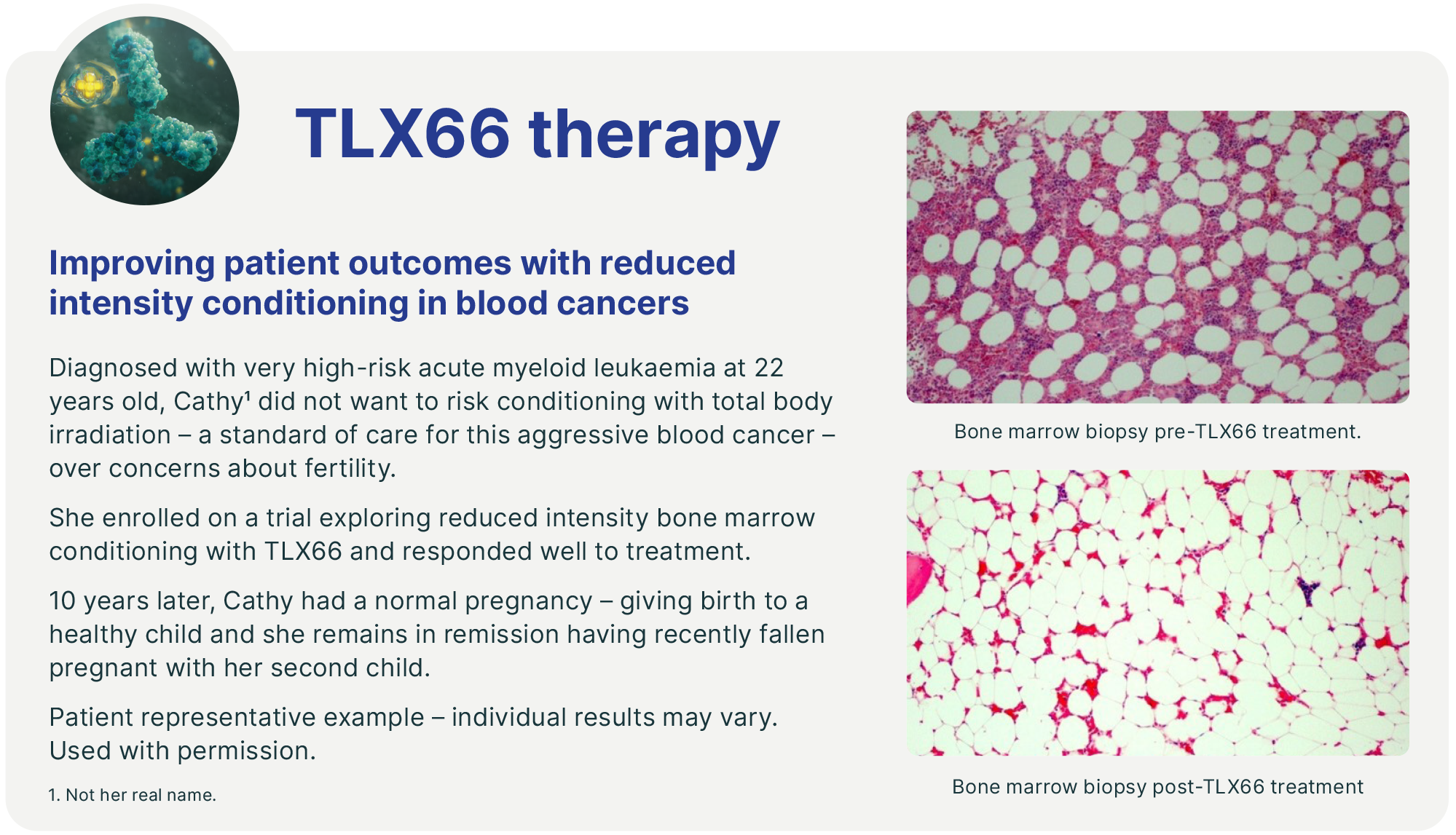
TLX66 (90Y-DOTA-besilesomab) – our investigational BMC therapy – has been evaluated in approximately 100 patients, with promising results both as a monotherapy and in combination with low-dose chemotherapy conditioning regimes.
We plan to evaluate TLX66 in a Phase II clinical trial as a BMC agent in patients with acute myeloid leukaemia (AML) who are not suitable for conventional BMC regimes. TLX66 is also being studied in multiple myeloma (MM), systemic amyloid light chain amyloidosis (SALA) and paediatric leukaemia through IITs. Clinical data suggests TLX66 could be a well-tolerated (and therefore highly versatile) BMC agent, which could be utilised as a single agent or in combination with either reduced or high intensity conditioning agents preceding either autologous or allogeneic HSCT.

Therapy
TLX66 is our investigational therapy for BMC for HSCT conditioning – a broad clinical indication with applicability to many different diseases.
Key TLX66 attributes include:
minimal uptake in non-haematopoietic organs, such as the liver, kidneys and gut
approximately 100 patients treated in several Phase I and II IITs in different haematological diseases (AML, MM, SALA) requiring autologous or allogeneic stem cell transplantation, and
ODD granted in the U.S. and Europe for BMC.
Imaging
TLX66-CDx is our imaging agent for osteomyelitis.
Key TLX66-CDx attributes include:
approved for imaging of peripheral osteomyelitis in 2010 by the European Medicines Agency, and
Phase III trial showed that imaging is accurate, efficacious and well-tolerated in diagnosing infection of the peripheral skeleton.

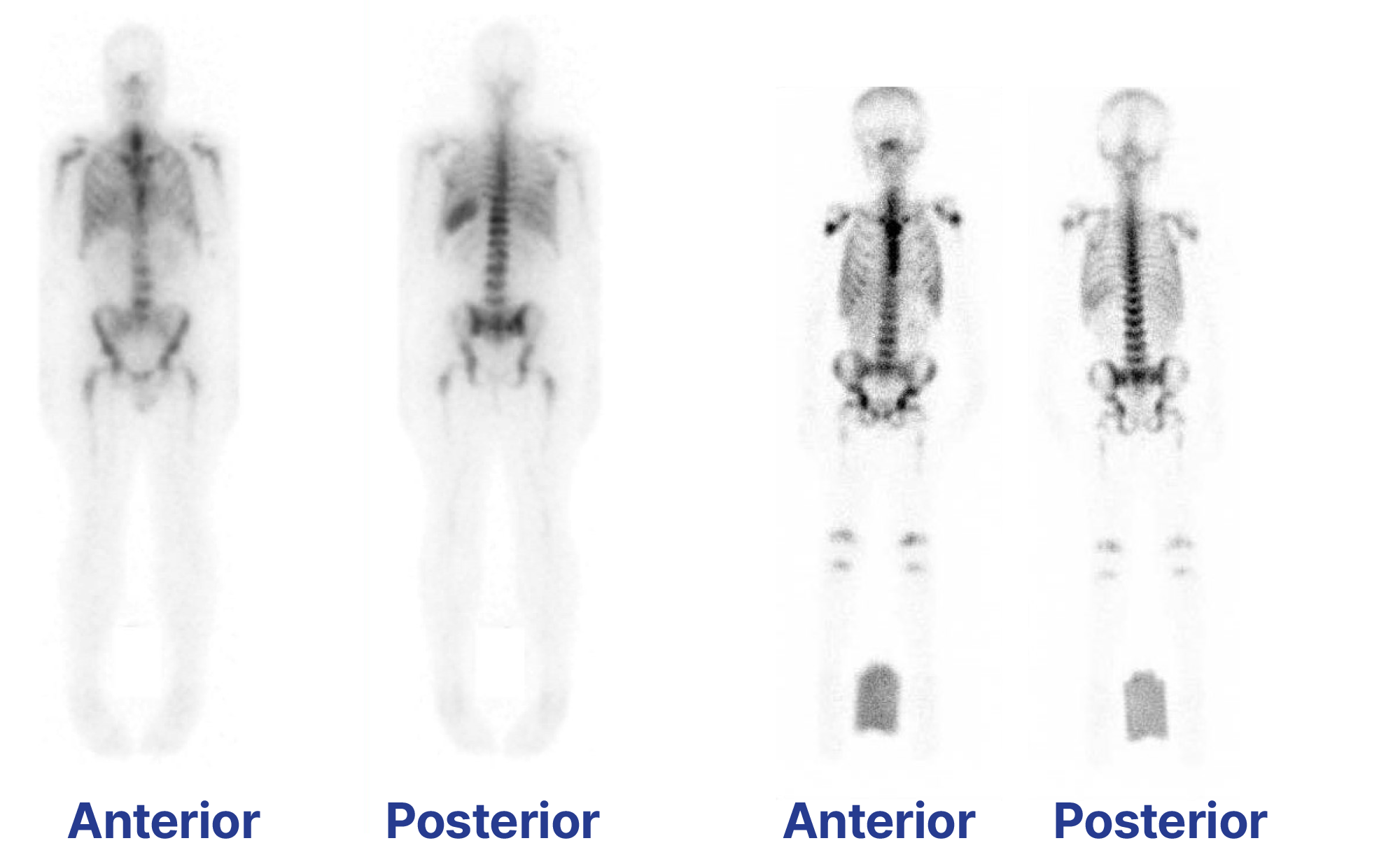
The target of TLX66 – cluster of differentiation 66 (CD66) – is a well validated leukocyte and neutrophil target. The imaging application of besilesomab (99mTc-besilesomab) has already been commercialised and is sold under licence by Curium Pharma as an approved product (marketed as Scintimun®) for imaging osteomyelitis (bone infection) in approximately 30 countries.
In parallel to the therapeutic applications of TLX66, we are exploring several indication expansions, as well as geographic expansion to key commercial markets.
Our precision-guided surgical solutions and artificial intelligence
During 2023, we established a new MedTech Division, further differentiating Telix by enabling us to create technologies designed to harness the power of targeted radiation across the entire patient journey from diagnosis to surgical intervention and therapy. We anticipate first applying this in urology – for prostate and kidney cancer – and then across the breadth of disease areas we are pursuing.
Radio-guided surgery
In November 2023, we acquired the SENSEI® radio-guided surgery (RGS) business from Lightpoint Medical.29 SENSEI® is a miniaturised surgical gamma probe for minimally invasive and robotic-assisted surgery. It is inserted into a surgical port and controlled by the surgeon during a procedure. When used with targeted imaging agents, SENSEI® may enable the intraoperative detection of cancer in real time, supporting greater precision in the removal of tumours.
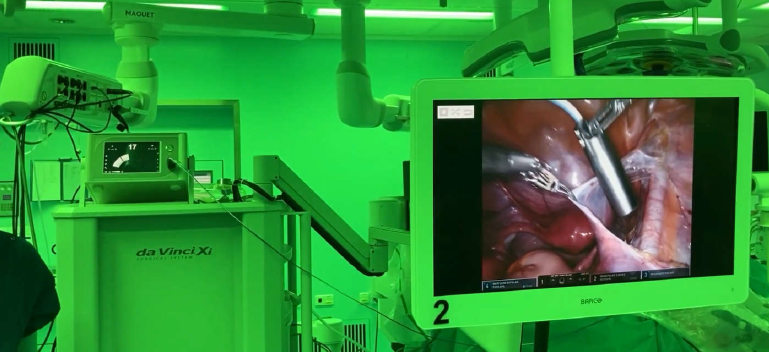
SENSEI® has attained a marketing authorisation in the U.S., having been registered with the FDA and has attained a Conformité Européenne (CE) Mark for use in the European Economic Area, for intraoperative detection of sentinel lymph nodes (SLNs).30
Our initial commercial objective is to align SENSEI® with our Illuccix® and TLX599-CDx (99mTc-iPSMA) programs for prostate cancer. Additionally, there is scope to expand the use of SENSEI® and explore advanced surgical radiation detection probes in other urologic and non-urologic malignancies, including TLX250-CDx (Zircaix™9), our kidney cancer imaging agent.
In November 2023, we made a strategic investment in Mauna Kea Technologies (Mauna Kea),31 a leading medical device company pioneering the development of real time intraoperative microscopic visualisation of tissue during surgery. This investment is an expansion of our existing alliance with Mauna Kea,32 established to develop new hybrid pharmaceutical device products through the combination of our cancer-targeting agents with Cellvizio®, Mauna Kea's confocal surgical laser endomicroscopy in vivo cellular imaging platform.
Cellvizio® is owned and marketed by Mauna Kea pursuant to multiple 510(k) clearances in the U.S. and is CE Marked for a range of applications in Europe.33

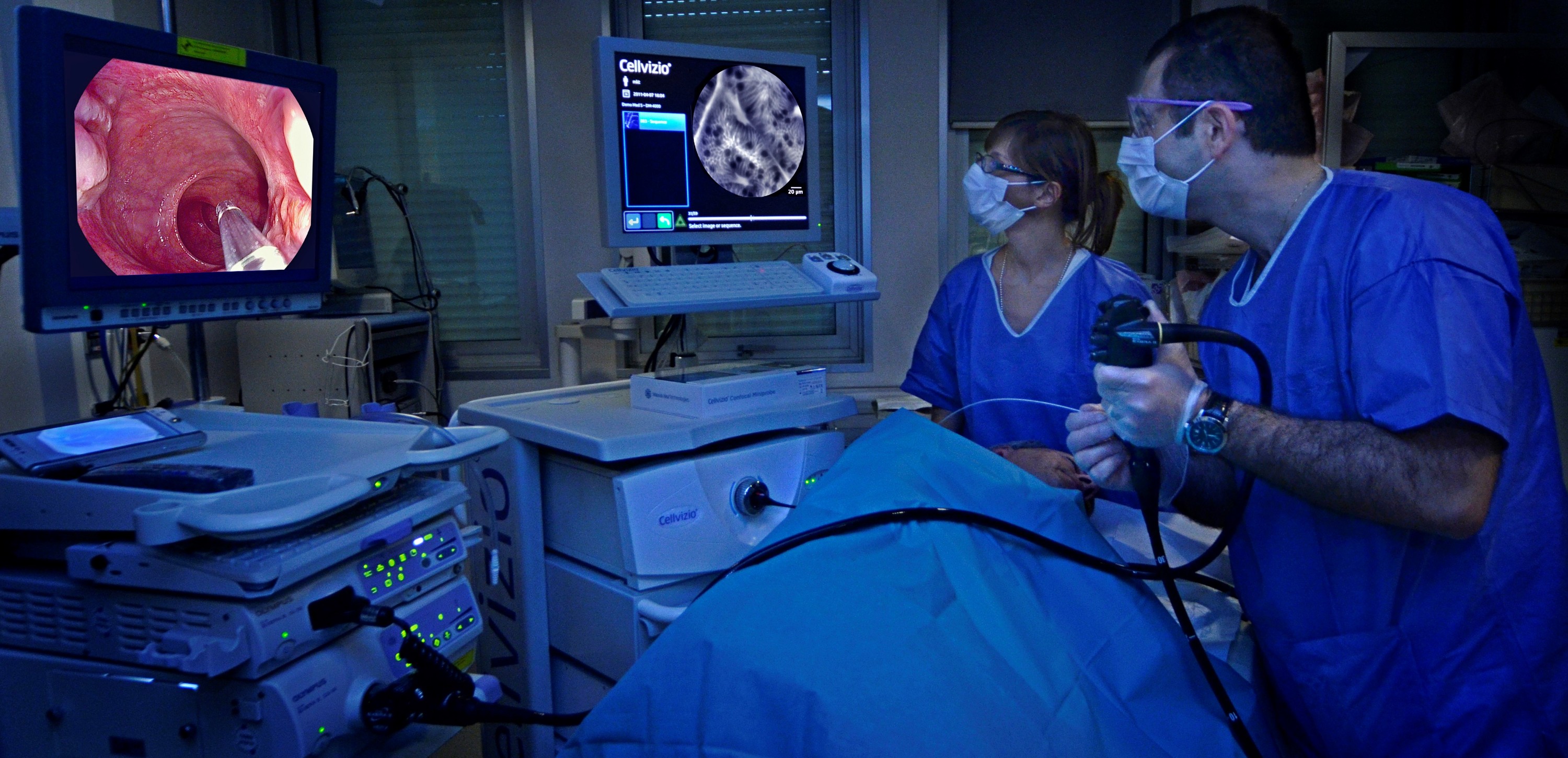
We believe this technology is complementary to our existing portfolio. When used preoperatively, our radiopharmaceutical imaging agents, such as Illuccix® or TLX250-CDx, allow for surgical planning to determine the location and extent of disease. SENSEI® works in conjunction with suitable cancer-seeking radiotracer agents to enable the intraoperative localisation of cancer in lymph nodes during a surgical intervention. In addition, Cellvizio® can be leveraged intraoperatively, in real time, to define and confirm surgical margin(s) via endomicroscopic fluorescence imaging.
Improving surgical outcomes by bringing molecular imaging into the operating theatre

Software as a Medical Device - powered by artificial intelligence
Medical devices and software applications are already an integral component in the delivery of nuclear medicine. Radiation dosimetry-planning software tools are used to inform therapeutic doses during development, and PET and SPECT cameras operate in conjunction with image-analysis software to interpret the resulting scan data. This data-rich environment supports the need for better software tools to drive clinical care and decision-making that can be enhanced with AI.
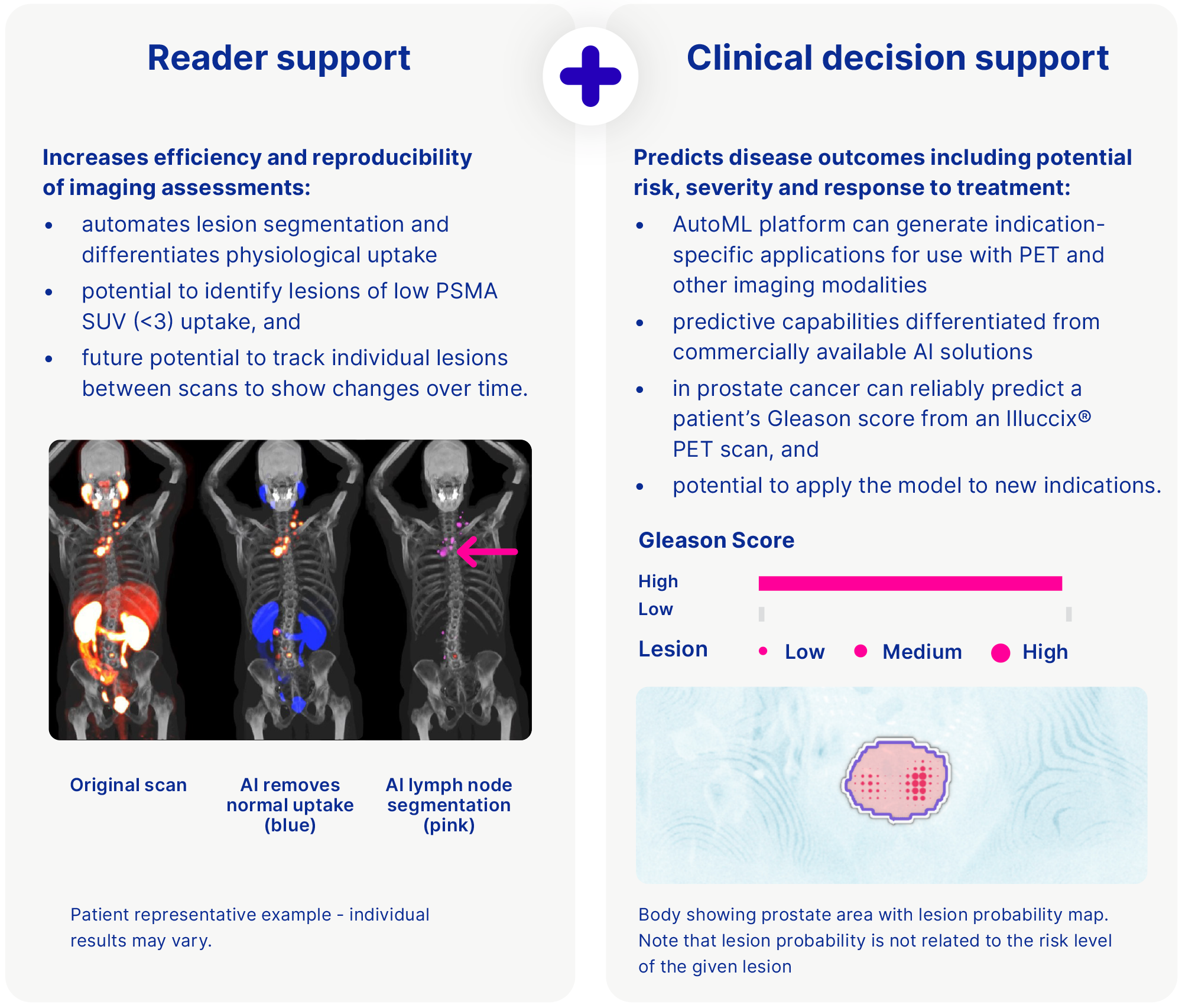
In 2023 we acquired Vienna-based Dedicaid GmbH and its clinical decision support software (CDSS) AI platform capable of rapidly generating indication specific CDSS applications from available datasets, for use with PET and other imaging modalities. Each CDSS application is trained to predict outcomes such as the severity of disease, risk to the patient and/or inform treatment decisions.
Dedicaid employs an automated machine-learning (Auto ML) engine. We believe that this platform is differentiated from commercially-available AI solutions currently used in PSMA-PET imaging, which are limited to supporting clinicians in the interpretation and reading of images – without a prediction capability. This platform is designed to reduce the time, cost and level of expertise required to build, test and validate new CDSS applications, facilitating a streamlined development and regulatory pathway for each new application.
Dedicaid developed the technology with PoC on the machine-learning methodology demonstrated for prostate, breast and lung cancer applications published in leading peer-review journals.34 We expect that our acquisition of this AI platform will provide us with the capability to quickly and easily generate algorithms from clinical data and medical images, add predictive capabilities alongside the imaging analysis module and will be used to accelerate the development of Telix AI applications across the pipeline. The Dedicaid acquisition also included a lead medical device tool that is designed to interpret the risk of prostate cancer advancement from a PSMA-PET scan image by correlating it to a well-known histopathology indicator (the Gleason Grade). A second AI asset supporting Illuccix®, being developed in partnership with Invicro LLC, is designed to automate the identification and classification of prostate cancer lesions from PSMA-PET scans to support greater efficiency and standardisation in the imaging workflow.
Telix AI: Earlier, more accurate diagnosis, and personalised disease predictions
The acquisitions of both Dedicaid and Lightpoint Medical’s RGS business provide a founding MedTech capability that we believe will enable Telix to generate AI and software applications that are complementary to our radiopharmaceutical pipeline.
Our research and innovation focus
Our research and innovation focus will define our future.
We are harnessing the power of targeted radiation to develop new targets, complement existing therapies and explore new clinical applications. Our aim is to build a pipeline of new product candidates and related platform technologies that can dramatically improve patient outcomes.
The team’s expertise in technology evaluation and product development – along with our standing as one of the world’s largest dedicated radiopharmaceutical companies – has opened up access to a range of new opportunities and partnerships.
Targeted alpha therapy
Alpha emitters have the potential to deliver very high amounts of energy to cancer tissue, while the short range can reduce the risk of damage to surrounding healthy cells, increasing the selectivity and potency of the radiation treatment.
Our vision is to develop alpha and beta therapies for the indications we are pursuing, to increase the options available to treat cancer within our portfolio and provide patients with additional options along their treatment journey.
For prostate cancer, we are developing TLX591 – a beta therapy, and the subject of the ProstACT series of studies – in parallel with TLX592, a potential alpha therapy currently being evaluated in the Phase I CUPID trial.
The TLX250 platform is also being explored as a potential alpha therapy in the investigator-initiated Phase II OPALESCENCE and Phase I PERTINENCE studies in breast and bladder cancer, respectively, which completed enrolment during 2023.
Immuno-oncology
An increasing body of scientific evidence suggests that low doses of targeted radiation can potentially overcome immune resistance. This approach, known as immunological ‘priming’, has the potential to render tumours more susceptible to cancer immunotherapy. Pre-clinical studies have shown an enhanced therapeutic outcome of checkpoint inhibitors when they are administered after a systemic radiotherapy,1 including rendering immunologically inert tumours sensitive to treatment.
The STARLITE-1 and STARLITE-2 Phase II IITs of TLX250 in kidney cancer therapy are evaluating CAIX-targeted radiation in combination with checkpoint inhibitors – a form of immunotherapy.
Tumour microenvironment
Tumours are complex, heterogeneous collections of cells. Their interaction with the surrounding microenvironment further enhances this complexity and can affect how the tumour grows and spreads. By better understanding the tumour microenvironment and harnessing the ability of targeted radiation to target multiple parts of the tumour, we are developing new approaches to complement existing treatments and make them more effective. An example of this translational research is our collaboration with Merck KGaA where we are exploring Telix’s targeted radiation in combination with Merck’s DNA damage repair inhibitors.
The Phase Ib STARSTRUCK trial is evaluating TLX250 in combination with peposertib – a Merck KGaA DNA-dependent protein kinase (DNA-PK) inhibitor candidate. The trial is evaluating the combination in patients with solid tumours expressing CAIX that are relapsed or refractory to standard of care treatment options. We believe that the combination may provide an enhancement in potency through the synergistic action on cancer cells. Targeted radiation effectively induces DNA damage in targeted cancer cells and peposertib may act to prevent the cell from repairing this damage, resulting in higher potency at lower doses.

We are working with leaders in the field to progress this research and have in-licensed a number of novel radiotracers for translation into new theranostics.
Novel applications of AI
Radio-imaging using targeted radiation relies heavily on digital data processing and input from highly trained technologists and radiologists to correctly interpret data. AI technology has the potential to transform image analysis by improving the accuracy and speed of decision-making for clinicians by recognising complex patterns in large datasets and conducting predictive analysis.
Telix is utilising its AI platform and AutoML capabilities to pursue disease diagnosis and risk prediction, and treatment response prognosis, and to investigate other advanced AI techniques to support and advance the clinical utility (and clinical trials) of its radiopharmaceutical products. This will provide clinicians with more information than is currently available to help make better decisions for their patients, leading to improved outcomes.
Surgical solutions
To fully explore the capabilities of the SENSEI® probe and role of Telix’s targeting agents in the operating theatre, we are exploring the use of different agents, alternative radioisotope and fluorescent payloads, and new detection technology to enhance surgical outcomes. This includes prototyping early drugs and devices for development as medical devices by the Lightpoint team.
Our manufacturing and supply footprint
During 2023, we made significant progress with the buildout of our radioisotope manufacturing facility in Brussels South, Belgium. At 2,800m2, Telix Manufacturing Solutions (TMS) is one of the largest radiopharmaceutical production facilities in Europe. The site will enable improved access to radiopharmaceuticals for patients across the Europe, Middle East and Africa (EMEA) region and worldwide as a primary good manufacturing practice (GMP) capable manufacturing site for our clinical and commercial products.

The site also has extensive R&D capabilities, with a focus on alpha-emitting isotopes. We believe the proximity of an alpha radiopharmaceutical laboratory (the 'AlphaLab') to a production GMP environment is a differentiated capability to our competition. We expect the site to evolve and develop as a hub for strategic collaborations via R&D facilities and a manufacturing line designated for university and small and medium-sized enterprise partners.

During 2023 we also completed the business integration of Optimal Tracers – a California-based company that provides radiochemistry process development services and research tracers for use in clinical trials. Optimal Tracers is advantageously located to service leading clinical sites along the West Coast of the U.S. and has the capability to deliver certain research products across the entire country. This expands our translational radiochemistry capability and offers a unique environment for pharma partnerships and collaborations.
- Pharma Intelligence September 2023, Prostate Cancer Disease Analysis report.
- Schwab M, ed. In: Encyclopedia of Cancer. 3rd ed.; Kratochwil et al. J Nucl Med. 2016; Wright et al. Urology. 1996.
- ClinicalTrials.gov ID: NCT04786847. Telix ASX disclosure 19 October 2023.
- ClinicalTrials.gov ID: NCT04726033.
- Tagawa et al. Cancer. 2019.
- Sung et al. CA Cancer J Clin 2021.
- Ljungberg et al. Eur Urol. 2019.
- Makhov et al. Mol Cancer Ther. 2018.
- Trade name subject to final regulatory approval.
- ClinicalTrials.gov ID: NCT03849118.
- Guzik et al. European Journal of Nuclear Medicine 2021; Patel et al. Science Translational Medicine 2021.
- Phase II STARLITE-1 IIT, ClinicalTrials.gov ID: NCT05663710.
- Phase II STARLITE-2 IIT, ClinicalTrials.gov ID: NCT05239533.
- Phase I STARSTRUCK study, ClinicalTrials.gov ID: NCT05868174.
- ClinicalTrials.gov ID: NCT04758780. Telix media release 7 December 2023.
- Telix ASX disclosure 18 October 2022.
- Telix ASX disclosures 7 November 2022.
- Sung et al. CA Cancer J Clin. 2021.
- Goodenberger et al. Cancer Genet. 2012.
- Ostrom et al. Neuro Oncol. 2018.
- ClinicalTrials.gov ID: NCT03849105.
- ClinicalTrials.gov ID: NCT05450744.
- Joint European Association of Nuclear Medicine/European Association of Neurooncology/Response Assessment in Neurooncology practice guidelines/Society for Nuclear Medicine and Molecular Imaging procedure standards for the clinical use of PET imaging in gliomas.
- Phase I IPAX-2 study, ClinicalTrials.gov ID: NCT05450744.
- Phase II IPAX-Linz IIT.
- In et al. Ther Adv Med Oncol. 2017.
- Telix ASX disclosure 17 April 2023.
- Neiderwieser et al. Haematologica 2022. An allogeneic haematopoietic cell transplant uses a donor's bone marrow or blood. The donor is usually a relative of the patient, although unrelated donors or umbilical cord blood are sometimes used. An autologous haematopoietic cell transplant uses a patient's own bone marrow or blood.
- Telix ASX disclosure 1 November 2023.
- Lightpoint Medical media release 20 January 2021.
- Telix media release 13 November 2023.
- Telix ASX disclosure 16 December 2020.
- Mauna Kea media release, 3 March 2020.
- Papp, L et al. Journal of Nucl Med. 2018; Papp, L et al. European Journal of Nucl Med and Mol Imaging. 2021; Zhao, M et al. European Radiology. 2022; Krajnc, D et al. Cancers. 2021; Papp, L, Journal of Nucl Med. 2019.
- Herrera et al. Cancer Discovery. 2022.