Purpose
Product and service safety, including clinical trial safety
Access to medicine
Patients. The reason we strive for excellence.
We conduct our clinical trials with a philosophy - and in a manner - that recognises the importance of patient safety and respecting the rights of participants.
We require that clinical investigators obtain and document informed consent - freely given without coercion - from all potential research participants.
We respect and maintain the privacy of research participants by ensuring individuals’ data is de-identified and we safeguard the confidentiality of each participant’s medical information in accordance with applicable laws and regulations.
Overall responsibility for product development, patient safety and related governance sits with our Group Chief Development Officer (CDO) and Group Chief Medical Officer (CMO). The CDO is responsible for ensuring decisions on product safety issues are made in the patients' best interests. The CMO is responsible for clinical trial development, ethical trial design, patient safety practices, and ethical conduct surrounding patient participation in clinical studies - paramount to clinical study development and conduct.
High-quality clinical research is conducted in accordance with all applicable laws and regulations. When conducting multinational, multi-site trials we follow legal, ethical and scientific standards, including Good Clinical Practice (GCP). The Guideline for GCP is an internationally accepted standard for designing, conducting, recording and reporting clinical trials. GCP captures informed consent for: clinical trial patients; trial management; data handling and record-keeping; clinical safety data management and reporting; and ethical conduct of clinical trials. We only conduct clinical trials when there is a sound unmet medical need and proven scientific methodology to investigate a scientific or medical question that is relevant to patients and/or healthcare professionals to improve patient lives and benefit society as a whole. We only enrol the number of participants required to answer the scientific questions under investigation.
To protect patient safety during the clinical trial process, we continuously collect, analyse, characterise and communicate the safety data of our products. We log adverse events as part of ongoing monitoring. We promptly share new findings and emerging concerns with investigators, regulators and/or research participants to appropriately manage risks associated with using our products, whether investigational or approved for marketing.
We have a Global Safety Review Committee (GSRC) that meets quarterly to oversee safety signal assessments across all Telix products in human use - including clinical trials, compassionate use, and post-market use - and collaborates on any actions needed. The GSRC comprises the CMO, medical affairs, pharmacovigilance, regulatory affairs, risk and compliance functional leads, and qualified persons.
In the event of a product quality issue that may impact patient safety, Telix’s Quality and Safety Evaluation Board (QSEB) is responsible for reviewing and evaluating product release and quality and safety issues. The QSEB comprises the CDO, CMO and senior representatives of the quality, regulatory, medical and risk and compliance functions. Outcomes of the QSEB are reported to the MD & CEO.
Improving access to medicine
Access to quality-assured medical products improves health and saves lives, but access is not equitable across the globe. One-third of the world’s population does not have access to essential medicines and treatments.
Our philosophy and statement of commitment are detailed in our Access to Medicine Policy. This includes our commitment to working with industry partners and patient advocacy groups to enhance access to medicine, particularly in the least developed countries or to the poorest populations in more developed countries.
Given the Group’s current size and stage of development, setting defined targets for access to medicine strategies is not appropriate. We will continue to assess this status as we grow.
We are committed to discovery and innovation; enabling access where possible, and incorporating compliant access strategies into our product development, post-clinical trial and lifecycle management plans and strategies; working with industry partners and patient advocacy groups with the aim of reaching more people with more products; and promotion of strong global healthcare systems.
We have a strong research and innovation program, with a strategic scope to develop new medical products, improve existing medical products and/or make them more accessible to patients with unmet medical needs worldwide.
Special, early or expanded access to investigational medicines ('Managed Access')
We believe participating in clinical trials is the most appropriate way for patients to be treated with investigational medicines prior to regulatory approval and marketing authorisation.
When participation in a formal clinical trial is not possible, patients with life-threatening conditions may, in some circumstances, seek special access to investigational medicines via a physician on a compassionate use basis. This is particularly the case for our CAIX-, PSMA- and LAT-targeting programs - for kidney cancer, prostate cancer, and glioma, respectively. These Managed Access programs are typically referred to as compassionate use, but can also be known as named patient request, magisterial prescribing, expanded access, early access and emergency use protocols.
Patient impact and expanded access
During the year Illuccix® and investigational products were used in 28 countries worldwide.
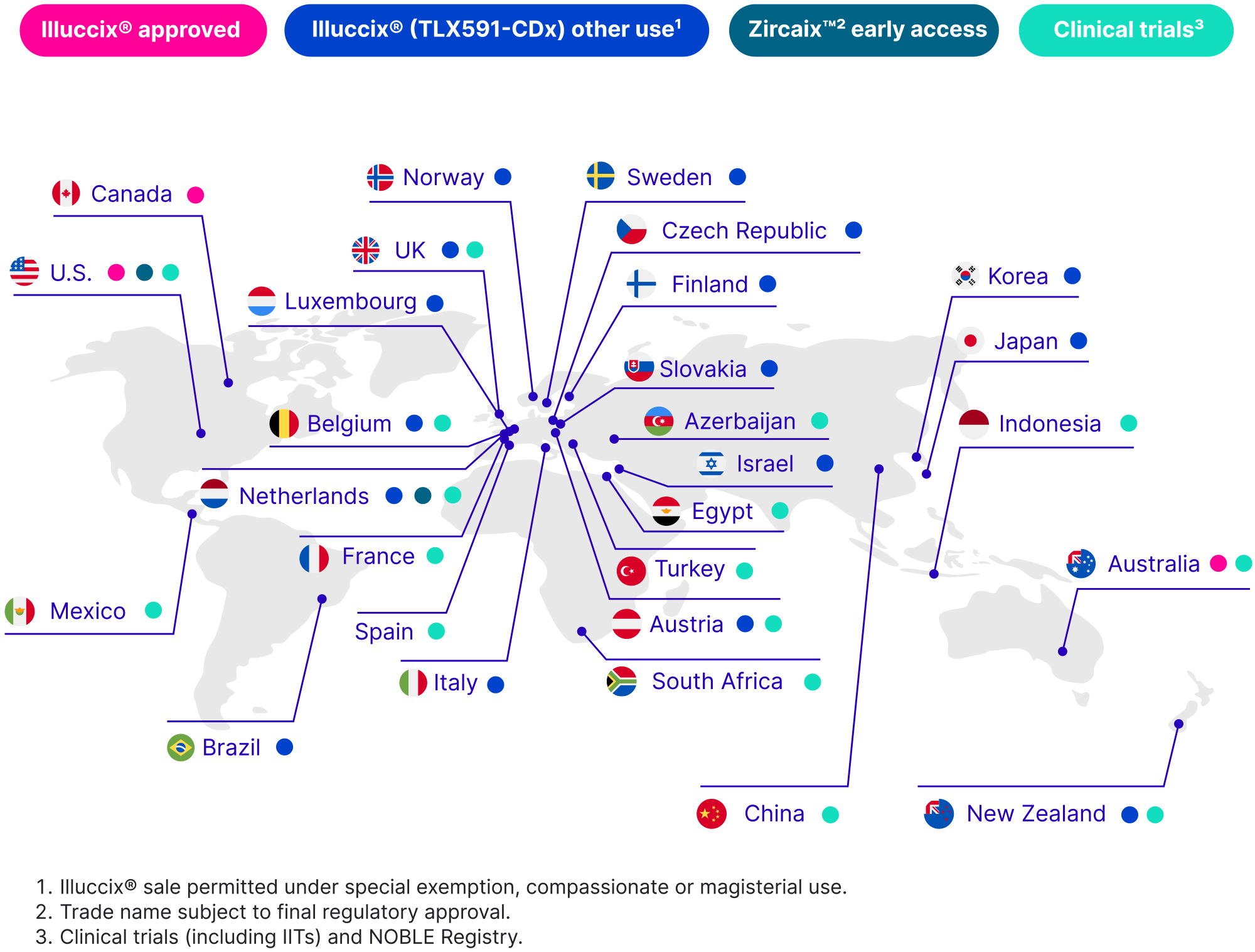
During 2023, Illuccix® became available in two additional regional Australian locations - Mackay in Queensland, and Hobart in Tasmania. For the first time, men living outside metropolitan areas in Australia have access to advanced prostate imaging that is both approved by the Australian Therapeutic Goods Administration (TGA) and available for reimbursement on the Medicare Benefits Schedule (MBS).
In September 2023, we launched a bursary scheme with RMIT University to support nuclear medicine students on placement in areas of rural, regional, and remote Victoria where there is a workforce shortage. Under the scheme, we will be making several placement bursaries available per semester for students across all year levels, to fund accommodation, travel and meal costs, and ensure that as many students as possible can access enriching education and experience outside major cities.

In October 2023, we formalised our support for the Oncidium Foundation’s radioligand therapy platform, RLT-Connect, aimed at reducing healthcare disparities, particularly in low- and middle-income countries. The platform connects healthcare professionals with radioisotope suppliers to make these therapies accessible to patients in need. This aligns with our core commitment to improve access to innovative radiopharmaceuticals globally.
In December 2023 we dosed the first patients in early access programs in the U.S. (ClinicalTrials.gov ID: NCT06090331) and the Netherlands for our breakthrough PET imaging agent TLX250-CDx (Zircaix™1) for the detection and characterisation of CAIX-positive lesions in adults with renal masses. This followed the completion of our successful global Phase III ZIRCON study, which reported positive results in November 2022, meeting all co-primary and secondary endpoints.2
Promoting strong global healthcare systems
We are actively involved in supporting policy and legislative measures that encourage innovation and promote access for patients, such as the FIND Act in the U.S..3 This U.S. legislation, which has growing bi-partisan support will ensure appropriate Medicare reimbursement and equitable access to the most innovative radiopharmaceutical imaging drugs.
People
Employee health and wellbeing
Diversity, equity and inclusion
Employee engagement, satisfaction and development
Everyone counts
Our success starts with our people. We are committed to providing a safe, healthy and inclusive workplace for our employees (including contractors) and have a comprehensive Health, Safety, Wellbeing and Environment (HSWE) strategy.
We comply with all applicable safety laws and regulations, and aim for the highest international standards in the harmonisation of our policies and practices across our operations. We seek to eliminate, as far as reasonably practicable, work-related injuries, illnesses and unplanned events from all aspects of our operations through programs related to our HSWE strategy.
Our global safety program is designed to drive a proactive safety culture and reinforce the link between our leadership behaviours and our HSWE strategy. We believe that through visible management, leadership and employee engagement, we can increase the awareness of potential risks and hazards and help employees make the right choices when it comes to HSWE.
HSWE leading and lagging statistics are reported to the GET, PCNRC, and Board. Statistics include incidents, accidents, near misses, training, wellbeing surveys, utilisation of the Employee Assistance Program, anonymous reports, hazardous environmental working practices and/or working practices that may impact the environment (considered from the context of employee wellbeing).
Our wellbeing program aims to advance the conversation on physical and mental health and support employees where and when they need it. The program is designed to help our people confidentially and proactively manage mental health concerns and challenges. Through the Employee Assistance Program, employees and their families can access early intervention and clinical resources, such as free, independent, confidential support from trained professionals.
We monitor and address employee wellbeing through regular surveys and initiatives to drive wellness, encourage work-life balance, and offer direct support for employees. We provide employees with up to four paid wellness days every calendar year (in addition to statutory leave requirements). We also promote 'meeting-free' days and, given the global nature of our operations and essential cross-jurisdictional collaboration, target zones for meeting versus meeting-free hours.
In 2023 we launched our Resilience First Aid program which identified individuals who are dedicated to promoting mental and emotional wellbeing within our organisation. These employees play a vital role in providing support, guidance and resources to help global colleagues build resilience, manage stress, and navigate challenges effectively.
Creating a safe workplace and culture that foster diversity, equity, inclusion, belonging and wellbeing drives a healthy, innovative and high-performing workforce. Cultivating a diverse and inclusive workforce, and fostering an environment that empowers wellbeing, helps us attract and retain top talent. Our programs and practices include:
hybrid work and flexible working
global paid parental leave policy and entitlement
‘Respect in the Workplace' training for all employees
mental health awareness surveys and initiatives, and
engagement surveys.
Developing our future leaders
We have a broad portfolio of internal and external learning and development opportunities available to employees at all levels. We provide internal development in the form of lunch-time webinars and seminars by subject matter experts, access to tens of thousands of self-paced online learning modules through the Learning Management System, and an opt-in ‘Learning Ladies Network’ available to all Telix learners.
In 2023, we launched the 'Rising Leaders' program offering 26 leaders the opportunity to develop their skills, mindset, and relationships to grow and thrive in their roles and prepare them for future leadership at Telix. The program includes participants from all regions and functions.
Investing in the professional growth and development of our diverse leaders and emerging talent contributes to diversity, equity, inclusion and belonging within our workforce. It also enhances our organisational agility and innovation while creating the next generation of leaders. As we continue along this path, we are confident that our empowered leaders’ diverse perspectives and skills will contribute significantly to our long-term sustainability goals.
We also support learning and development opportunities from external providers. During 2023, three of our female leaders completed the Australian-based 'Women Rising' program, which takes an evidence-based approach, grounded in psychology and neuroscience, to help female leaders reach their leadership potential. Opportunities can be identified by managers, People & Culture, or employees themselves and accessed through a structured application process.
We further support our employees through annual goal setting and performance reviews, annual performance-based bonuses, our equity-based incentive program and hybrid work arrangements. Employees can also apply to access the learning and development budget for leadership or business coaching. Our People & Culture plan for 2024 includes an inaugural mentorship program.
Combining people and purpose
In September 2023 (Prostate Cancer Awareness Month), Telix global employees participated in the Prostate Cancer Foundation of Australia’s The Long Run and ZERO Prostate Cancer’s Run/Walk in the U.S.. Collectively, the team raised more than $27,000 for prostate cancer education, testing, patient support, research and advocacy.
Gender diversity in the workforce
We are committed to advancing diversity – in all its forms – in the workplace. Gender diversity has been a focus throughout our history and women represent 49% of our global workforce. The Board and GET monitor the percentage of women in the workforce, with a particular focus on increasing female representation in senior management.
Our gender representation progress through the 2023 financial year is as follows:
we have met the gender representation goal set by the ASX Corporate Governance Council, of at least 30% of each gender on the Board, with employees identifying as female represented at 33%
the representation of women in our Global Leadership Forum is at 44%, and
women represent 30% of our Band 3 employees.
See our Corporate Governance Statement for more information available at www.telixpharma.com/investor-centre/corporate-governance.
Principles
Business ethics and integrity
Transparency and reporting
Supply chain responsibility
Labour practices and human rights
We act with determination and integrity
We have established policies and procedures, including our Code of Conduct, that articulate our principles and values and provide a framework for ethical conduct. Our Code of Conduct establishes our expectation that management, employees, and agents of Telix act in accordance with all applicable laws and Telix policies and procedures, as well as the highest standards of ethics. The Code of Conduct emphasises a strong culture of integrity and ethical conduct in association with independent Anti-Bribery and Anti-Corruption and Whistleblower Protection Policies.
We have policies applicable to interactions with healthcare professionals both in the public and private sector, and all interactions are additionally expected to comply with applicable laws. These policies, including the Code of Conduct, detail multiple reporting channels for suspected breaches and are strongly linked to the Whistleblower Protection Policy.
Material breaches of the Code of Conduct and the Anti-Bribery and Anti-Corruption Policy, and reports of incidents under the Whistleblower Protection Policy, are reported to the Board. This program is periodically reviewed for its effectiveness and promoted to all employees.
We have a global ethics and compliance program designed to promote compliant and ethical conduct and to prevent and detect violations of the law and our policies.
All employees are required to undertake compliance training as part of induction and at regular retraining intervals. This training covers our Code of Conduct and key policy areas relating to anti-bribery and anti-corruption, modern slavery, privacy, competition, whistleblower protection, diversity, equity and inclusion, anti-discrimination, and workplace health, safety and wellbeing.
Our employees are expected and encouraged to speak up, ask questions to seek guidance or clarification, and report ethical or compliance concerns in good faith and without fear of retaliation. Clear instructions about this are addressed in our Whistleblower Protection Policy, which is available in English, French and Japanese – the key employee languages used across our business. We have a commitment to no retaliation, which is documented in our Whistleblower Protection Policy.
We have internal and external reporting channels, including anonymous options, for employees, suppliers and other relevant parties to report reasonably suspected misconduct, compliance violations or other matters, including:
unethical/illegal behaviour
coercion
harassment or discrimination
modern slavery
privacy
fraud or corrupt practices, and
workplace safety or environmental hazards.
Our grievance, complaints and reporting procedures all include clear processes for investigating and responding to claims and concerns in an ethical, confidential and transparent way.
We also raise awareness of important global community issues via internal events and programs, such as International Women’s Day, Pride Month, World Suicide Prevention Day, R U OK? Day, World Mental Health Day, International Human Rights Day, and Movember.
Supply chain responsibility and transparent reporting
We expect our employees and relevant business partners to adhere to our values and commitments, wherever they operate. We strive to have a transparent supply chain and to report in a way that complies with all applicable modern slavery and human rights legislation.
Through our Supplier Code of Conduct, we have documented the expectation that suppliers implement their business in a manner that respects and supports human rights. We expect that they:
do not engage in or tolerate activities that contribute towards human exploitation, including as relates to children and child labour
respect and support worker needs with regard to wages, benefits and working hours
respect workers' rights to freedom of association and privacy
do not engage in or tolerate discrimination in any form
comply with health and safety laws
actively manage the environmental impact of their operations, and
conduct their business in a fair, ethical, professional and transparent manner.
We support and track adherence to our Supplier Code of Conduct with high-risk suppliers in a multitude of ways:
building awareness and supporting suppliers to address modern slavery and human rights risks in their operations
minimum contractual clauses for compliance with applicable laws
building awareness within employee base
utilising data from complaints/grievance mechanisms to assess the effectiveness of systems, policies and practices
delivering compulsory training for all staff and targeted training opportunities
utilising internal and external audit programs to verify the effectiveness of internal controls, and
quarterly risk reporting to the GET and ARC.
We collect and monitor internal and external information for use as data points in line with applicable laws to inform and improve the definition of our supplier risk profile, and support our human rights risk assessments.
The majority of products and services required for our business or supply chains are procured from Australia, the U.S. and countries in Europe - countries that do not create inherent heightened modern slavery risks (2023 Global Slavery Index).
However, we recognise that modern slavery risks may exist in partner supply chains that may not be obvious from the country source of such products or services. These risks generally relate to a lack of awareness and acceptance of modern slavery risks or where there are underdeveloped human rights or labour laws. This is particularly relevant for small to medium-sized enterprises still developing their management capability and understanding of labour standards.
There are limitations in our ability to influence our broader supply chain, but we engage with our key direct suppliers on a risk basis to raise awareness of modern slavery risks within their own organisations and supply chains.
While we recognise that spend does not necessarily correlate with modern slavery risk, we have included it as a relevant risk analytic because of our assumed increased ability to influence our high-spend suppliers to address this risk.
We use a range of internal and external data sources in supplier onboarding and due diligence processes to continually improve the definition of supplier risk profile.
In 2023, we continued to identify and refine the traceability of goods and services within our own and our supplier operations, focusing on the geographic scope and goods and services most relevant to our business.
We acknowledge that without adequate contractual arrangements and due diligence there is a risk that we could contribute or be directly linked to modern slavery practices through our arrangements with suppliers. We take the following precautions to help verify that our suppliers meet our expectations:
we select suppliers based on their merit and quality of goods or services
before contracting suppliers we consider to be of higher risk, and periodically after that, we conduct targeted, risk-based due diligence through a tiered, risk-based program
we include targeted provisions in supplier contracts to request compliance with applicable laws and principles of relevant Telix policies, and
we require Telix pre-approval for any sub-contracting for key suppliers.
Labour practices and human rights
We respect human rights and are committed to creating a safe workplace with a culture that fosters diversity, equity, inclusion, belonging and wellbeing. We are committed to operating our business with integrity and accountability, including respecting worker rights, complying with employment and human rights laws, and working to prevent any child labour, modern slavery or human trafficking from occurring in any part of our business operations or supply chain.
Our philosophy is based on and informed by the United Nations’ (UN) Universal Declaration of Human Rights, the UN Guiding Principles on Business and Human Rights, and the International Labour Organisation’s Declaration on Fundamental Principles and Rights at Work. During 2023, we published our inaugural Modern Slavery Statement for the 2022 financial year.
In Australia, Telix is subject to the requirements of the Payment Times Reporting Scheme which requires us to publicly report on payment terms and practices for our Australian small business suppliers. During 2023, we received a total of 591 invoices from 73 small suppliers and based on the invoice value, paid a total of 90% within 60 days of receiving the invoice, with 66% being paid within 30 days.
Ethical use of animals
We are involved in testing potential new medicines on animals and in humans. This is an essential requirement of international medicine development and regulatory approval processes. Telix has and enforces an ethical use of animals policy that requires all studies undertaken involving animals or humans are developed in association with medical, scientific and regulatory advisors. These studies reference national and international ethical and scientific codes, including Australia’s National Health and Medical Research Council and the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. Studies are only undertaken when there are no alternatives, represent the only feasible option to advance investigational agents, and only commence after necessary ethics approvals have been received from the institution or clinical site at which studies are to be carried out.
Performance
Risk management
Data privacy and cybersecurity
Board composition and governance
Executive compensation and benefits
We strive to be extraordinary
Effective risk management is essential in delivering sustainable value for our stakeholders. It requires commitment and involvement across the business, from the Board to employees at all levels of our operations. The Managing risk section in this Report provides a comprehensive overview of our risk management processes and procedures.
Protecting privacy and ensuring information security
We are committed to protecting the privacy of all individuals with whom we engage. Telix’s global Privacy Policy describes how we collect, use, disclose, protect and store personal information collected, and what choices and rights individuals have with respect to that information. We do not generally collect ‘sensitive information’ (defined as including, for example, information about racial or ethnic origin, political opinions, religious beliefs or affiliations, membership of trade unions or associations, and sexual preferences or practices), other than health information in very limited circumstances in relation to a clinical trial, or reasonably necessary to ensure the health and safety of personnel at Telix premises around the world.
We take all reasonable steps to ensure the security of our systems and to protect information from misuse, interference and loss, as well as unauthorised access, modification or disclosure. We limit access to personal information. Information is stored on high security servers. In the event of a data breach, we are committed to complying in all respects with the requirements of all relevant privacy laws, including, but not limited to, the provisions of the Australian Privacy Act, the General Data Protection Regulation (GDPR), the UK Data Protection Act, the U.S. Health Insurance Portability and Accountability Act (HIPAA), the Californian Privacy Act and the Japan Act on the Protection of Personal Information (APPI). We have in place data breach policies and plans that apply when handling personal information breaches under applicable laws.
We have an Information Security and Information Management (ISMS) program led by the Chief Information Officer and Director of Cyber Security. Our policy is that information in all forms must be protected from accidental or intentional unauthorised modification, destruction or disclosure throughout its lifecycle. This protection includes an appropriate level of security over the equipment, processes and software used to process, store and transmit information. We have established and seek to continuously improve effective information security governance. We adopt a risk-based approach in line with the ERMF to address potential gaps in security controls. All employees must participate in information security training when hired, with at least annual refresher training.
We conducted an internal audit of our ISMS system during 2023 to assess effectiveness and associated compliance.
Structuring the Board to add value
The Board is committed to ensuring that it comprises individuals who collectively have the appropriate skills and experience to develop and support its responsibilities and Company objectives. See our Corporate Governance Statement available at www.telixpharma.com/investor-centre/corporate-governance for further information on the Board’s composition, role and responsibilities.
We promote Director and employee ownership of shares to foster shared ownership and commitment to company, stakeholder, partner and patient outcomes. All Directors own shares in Telix, and the Company utilises an Employee Incentive Plan to encourage and enable share ownership by all employees across the organisation.
Our performance during the year – linked to executive remuneration
See 2023 highlights section of this Report for a snapshot of financial and operational performance. Additional detail on business performance can be found in the Our performance, strategy and future prospects section of this Report.
Planet
Environmental sustainability
Climate strategy
Human health is interconnected with the health of the planet
Climate change is a global issue leading to an ever-growing responsibility of companies and industries to understand and address their environmental impact and sustainability. We are committed to contributing to the creation of a more sustainable world.
We monitor existing and emerging risks stemming from the manufacturing, regulatory, people and supply chain aspects of our business.
We recognise that our operations and business activities could affect biodiversity values within land, marine and freshwater ecosystems. We aim to operate in a manner that will minimise our environmental impacts and promote sustainable land use. We will apply a mitigation hierarchy of avoidance, minimisation and mitigation during the lifecycle stages of our operations.
We are committed to meeting international standards in biodiversity protection. We believe in science-based solutions and will form partnerships to better understand potential impacts and inform management practices.
We are committed to maximising natural land use on our sites and minimising biodiversity impacts. Our plans for natural land best use and regeneration include tree-planting and preserving or restoring habitats that promote biodiversity activity – wherever possible, and in accordance with local regulation and guidance.
We will conduct environmental risk assessments while we explore the feasibility and practicality of implementing a formal Environmental Management System. As part of this process, we aim to balance the delivery of Telix’s strategic objectives and obligations under the complex regulatory environments in which we operate.
We follow the practice of ’plan – do – check – act‘ as we continue to:
review our environmental goals
analyse the environmental impacts and legal requirements related to our operations
set objectives to reduce environmental impacts and comply with legal requirements
establish programs to meet these objectives and targets
monitor and measure progress in achieving the objectives, and
seek to ensure employees’ environmental awareness and competence.
Nuclear industries must carefully monitor and control what they release into the environment to keep the air, water and land clean. We recognise and support the safety standards of the International Atomic Energy Agency (IAEA) and the International Commission of Radiological Protection (ICRP), which provide rigorous regulatory mechanisms to restrict the release of radionuclides and control any radiological impact on people and the environment. We are committed to limiting the release of radioactivity into the environment and ensuring compliance with established radiation protection standards.
We will apply strategies, processes, practices and procedures to effectively manage the safe manufacture, transport, disposal and waste management of radiopharmaceutical products relevant to our business operations and activities. We will ensure waste management and disposal infrastructure is established and maintained, and we will maintain accurate and complete records for reporting purposes to nuclear regulatory authorities in the jurisdictions in which we operate. We will also apply and promote procedures for the responsible manufacture, transport, disposal and waste management of radiopharmaceutical products, and acknowledge and reward employee innovations that enhance our performance in this area.
We have a rigorous assurance program through which we conduct due diligence on and internal audit of material vendors and suppliers. This includes ensuring our relevant vendors and suppliers have the appropriate licences and SOPs, and regulatory compliance certifications (where required) for the safe disposal of radiopharmaceuticals.
Principles in practice
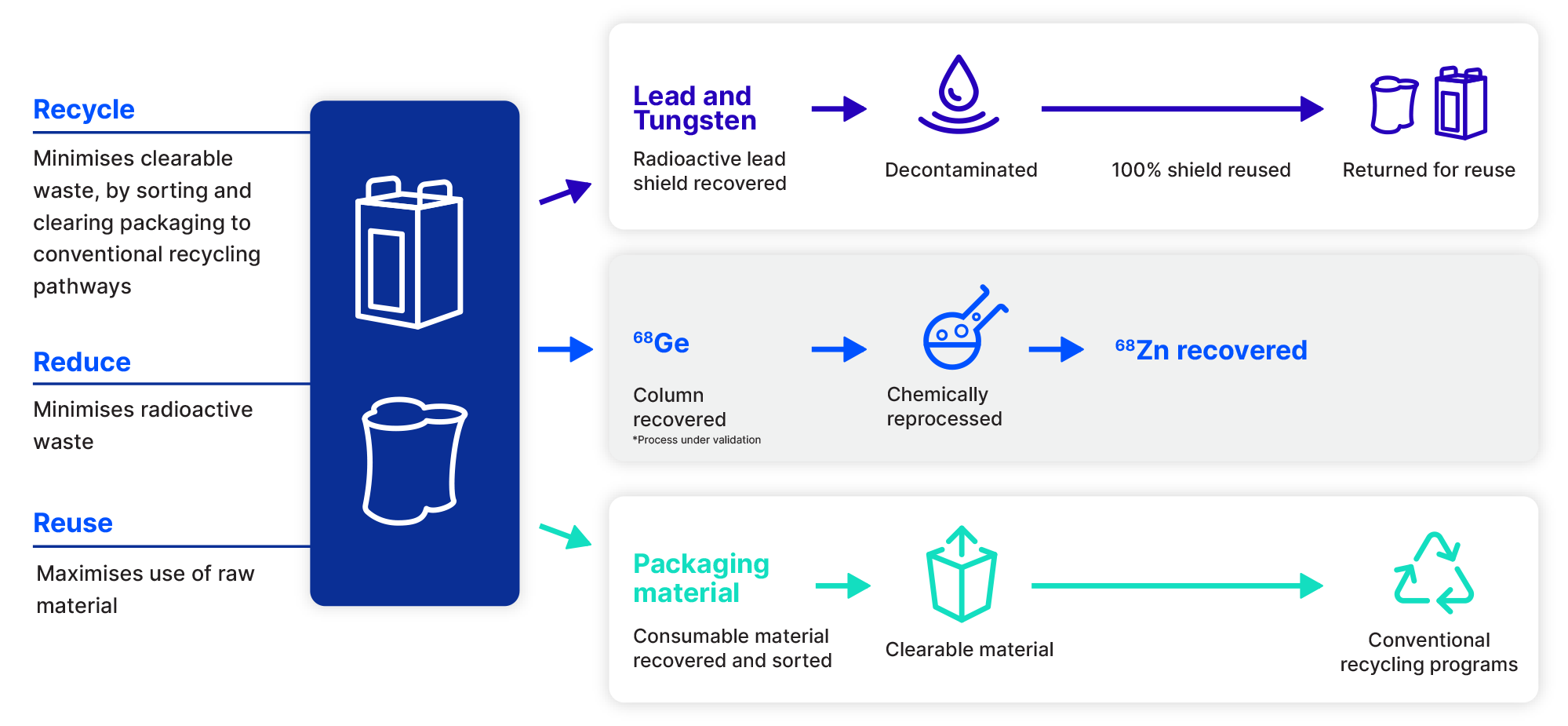
The Illuccix® kit is intended for use with Eckert & Ziegler’s GalliaPharm® and IRE EliT’s Galli Eo® gallium-68 generators, where, in both cases, the three main components can be recycled. The radioactive lead shield is recovered, decontaminated and returned for re-use. The germanium-68 column is recovered and then chemically reprocessed to recover zinc-68 (85-95%) for recycled zinc-68 targets. Packaging is separated into consumable material (recovered and sorted) and clearable material, which can enter conventional recycling programs.
Recycle, reduce, reuse 68Ge/68Ga generators
Contribution to our local and global sustainability goals
Climate related disclosures
We support the Task Force on Climate-related Financial Disclosures framework, which we have used to guide our 2023 climate-related disclosures. We also support the International Sustainability Standards Board's (ISSB's) issuance of its inaugural standards: IFRS S1 and IFRS S2. We continue to develop our disclosure methodology in accordance with leading standards and frameworks, including the emerging Australian Sustainability Reporting Standards – Disclosure of Climate-related Financial Information.
Informing the Board about environmental issues and climate change
We identify and manage environmental and climate-related risks and opportunities as part of, and in accordance with, our ERMF – including when determining the materiality of our exposure to climate-related risks. We are working to integrate climate risk into the supporting policies, processes and controls for our key climate risks and continue to update these as our climate risk management capabilities mature.
We operationalise the three lines model for risk and opportunity management and assurance. Each functional lead is the ultimate first-line risk and opportunity owner for their Business Unit. With leadership, guidance and support from the second-line GRC function, the functional leads form the sustainability committee within the business, ensuring that sustainability and climate- related matters are considered when setting and implementing Business Unit strategy, objectives and activities.
Climate strategy is governed by the Board, while our operational climate guidance and day-to-day management of climate- and sustainability-related risks and opportunities are driven by management. The Board and its committees receive quarterly presentations from management on environmental, governance and social issues, such as climate; diversity, equity and inclusion; pay equity; and the integration of sustainability factors into our operations. The ARC receives quarterly reports on material risks and opportunities to the business, including environmental and climate-related matters.
Management reports the status of initiatives addressing environmental risk (strategy implementation, target setting, policy and other indicators, and risk response) to the ARC. During 2023 we delivered against a strategic plan to start capturing and recording data (including data integrity and assurance processes) to help us understand our environmental footprint and inform our goals for disclosure and future setting of science-based targets.
Climate-related issues that may have a material financial impact on the organisation
Understanding the potential impact of future climate scenarios - together with proactive mitigation, intervention plans and targeted investment in line with our overarching strategic objectives - is an essential consideration of the Board and management. This understanding supports our efforts to build resilience and ensure long-term sustainability and continued development and commercialisation of theranostics for patients living with cancer and rare diseases.
We are also committed to understanding the physical climate change risks for our workforce in delivering on our purpose.
In 2023 we focused on identifying environmental and climate-related risks and opportunities. Our priorities for 2024 and 2025 include screening climate impacts across our operations on a risk-assessed basis and defining a methodology to ensure climate risks associated with our operations are integrated into our business planning.
We will use scenario analysis to help us understand the broad range of climate-related issues that may impact our business. Our focus will be on enhancing the resilience of our operations while implementing energy efficiency initiatives and renewable energy projects where possible, practicable and relevant to the delivery of our strategic objectives. We will aim to incorporate applicable physical climate scenarios into supply chain, built assets, and operational decisions.
We are developing strategies and plans to increase our knowledge base with regard to the potential financial impact of extreme weather events (e.g., the cost of supply interruptions), to enable us to develop appropriate mitigation and intervention plans. We will assess these financial impacts and disclose them where material.
In 2024 and 2025 we aim to develop methodologies that consider the potential impact of extreme weather events on our business, strategy and financial planning in the following areas:
products and services
supply chain and value chain
adaptation and mitigation activities
investment in R&D
operations
acquisitions or divestments, and
access to capital.
The nature of the risks and opportunities we face depends on the physical aspects of climate change, as well as transition risks related to regulatory and commercial changes in the markets in which we operate. In addition to these risks, there is the opportunity to reduce our carbon footprint and to shape a culture of climate action focused on de-carbonising our value chain. Physical and transition climate-related risks may include:
Risk or opportunity | Description | Mitigation and management |
|---|---|---|
Policy and legal risks | There is uncertainty over the future environmental policy and fiscal landscape in many countries where we operate. We anticipate increased regulation and other developments related to carbon pricing and broader environmental taxation over the medium to long-term. We may face increased pricing of greenhouse gas (GHG) emissions, enhanced emissions-reporting obligations, mandates on and regulation of existing products and services, which may in turn increase our exposure to litigation. | We aim to maintain awareness of current and emerging policy across our areas of operation. This includes proactive searches, participation in industry and policy area events and conferences, subscriptions and memberships to policy monitoring services, and engagement of expert third parties for advice. We will continue to incorporate awareness of emerging issues into our business sustainability thinking and decision- making. We aim to understand our GHG footprint and set targets to reduce or offset our emissions where applicable. We will look to implement life-cycle assessments for products that include a GHG footprint to help assess and manage risks to reduce the environmental footprint of our products. We will aim over time to develop an asset sustainability index to capture GHG (Scope 1, 2, 3) per product per patient per annum, as well as targeting the measure of percentage of renewable power and other resources used to make that product. |
Market risk including changes in cost base and changes in technology | Separate to supply chain resiliency, increased operational costs in the supply chain may have an effect on pricing and costs of raw materials including packaging and logistics. We may face changing customer behaviour, uncertainty in market signals and/or increased cost of raw materials. | We aim to continuously develop our understanding of our critical supply chain and value chain so we can address emerging risks. We recognise the opportunity to develop products with lower emissions ratings that may connect to higher commercial potential. We will continue to use financial risk management strategies to manage increasing costs associated with broad market changes. We recognise that more efficient buildings will reduce costs and improved facilities management will lead to lower costs for repair and replacements. The use of lower-emission sources of energy will reduce costs and will reduce exposure to fossil fuel and carbon price changes. The use of more efficient production and distribution processes will reduce operational and logistical costs. Each of these may contribute to offset cost increases elsewhere in the value chain. |
Increased frequency of extreme weather and climate-related natural disasters (acute physical risks); long term changes in climate (chronic physical risks) | Acute climate-related risks may manifest when physical risks, such as flooding or storms disrupt our own properties, operations, rented corporate service arrangements and/ or material supply chain partners. Chronic climate-related risks can come from long-term changes in precipitation, extreme variability in weather patterns, rising mean temperatures and/or rising sea levels. | We will seek to mitigate material business impact arising from short-term events by investing in at-risk Group sites, through thoughtful design and sustainability of supply chains, and by managing levels of inventory. We will address identified risks in business continuity plans and will integrate required investments into the normal mid- and long-term financial planning process. We will assess and determine suppliers with high criticality and exposure to significant future climate hazards, and aim to work with them to ensure that they build climate-related resilience into their business continuity plans. We will conduct climate risk assessments in site evaluation criteria for investment in new operations. Managing long-term changes in climate will require a proactive and strategic approach. We are committed to understanding the science of climate change, including its potential impacts, and taking action relevant to managing risk associated with our operations. This will include adaptation planning and may include, for example, building or purchasing climate-resilient infrastructure. |
- Trade name subject to final regulatory approval.
- Telix ASX disclosures 7 November 2022.
- Facilitating Innovative Nuclear Diagnostics Act (H.R 1199).